More Information
Submitted: September 04, 2023 | Approved: December 14, 2023 | Published: December 15, 2023
How to cite this article: Kanoute A. Epidemiological and Clinical Aspects of Staphylin Dermatitis: A Study of 30 Cases at the Dermatology Hospital in Bamako. Ann Dermatol Res. 2023; 7: 036-039.
DOI: 10.29328/journal.adr.1001029
Copyright License: © 2023 Kanoute A. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Epidemiological and Clinical Aspects of Staphylin Dermatitis: A Study of 30 Cases at the Dermatology Hospital in Bamako
Abdoulaye Kanoute*
Department of the Dermatology, Hospital in Bamako, Mali
*Address for Correspondence: Abdoulaye Kanoute, Department of the Dermatology, Hospital in Bamako, Mali, Email: [email protected]
Staphylin dermatitis is an acute, irritant, contact dermatitis caused by Pederin, a haemolymphatic fluid released when staphilinidae are crushed against the skin [1]. This is a serious condition, with epidemics and certain localizations, especially in the eyes, which can lead to blindness.
The Staphylinidae family comprises over 622 species. In West Africa, Paederus sabaeus is the most widespread species [2].
Species distribution varies according to eco-climatic zone.
In tropical environments, they proliferate at the end of the rainy season.
These insects are more active in the evening and are attracted by artificial light in the evening [2-4].
Epidemics were reported in Peru in 1998 and 1999 [5].
In Africa, around 1/3 of the population of the Kenyan capital has been affected by staphylin dermatitis [4].
Outside epidemic contexts, staphylin dermatitis has been described mainly in expatriate populations.
In Mali, staphylinidae have been described by entomologists in the Sikasso region [2].
To our knowledge, no studies have been carried out on insect-induced dermatitis.
The aim of this study was to describe the epidemioclinical aspects of staphylin dermatitis in the Dermatology Hospital of Bamako.
Setting and study site
The study was carried out at the Dermatology Hospital in Bamako (HDB).
Type of study
This was a descriptive cross-sectional study of cases of staphylin dermatitis.
Study period
This study took place over a period of 3 months (October to December 2022).
Study population
It was represented by patients consulting the Dermatology-Venereology department at the Dermatology Hospital in Bamako.
Inclusion was exhaustive.
All ages were accepted at inclusion. The diagnosis of staphylin dermatitis was clinical. It was defined by the presence of bullae and or erosion localized in the uncovered areas accompanied by functional signs.
Inclusion criteria
All patients meet the case definition.
Non-inclusion criteria
Patients not consenting or unable to give consent.
Study design
Cases were recruited during dermatological consultations. A general examination followed by a dermatological examination was performed. Patients were reviewed 10 days after inclusion. Sociodemographic, anamnestic, clinical, and evolutionary data were recorded on a survey form.
Data entry and analysis
The data were entered and analyzed using Epi info version 7 French. This was a descriptive study and no statistical analysis was performed.
Ethical aspects
Informed consent was obtained prior to inclusion. Inclusion did not involve any additional risk for the cases. The anonymity of the cases was guaranteed.
During the study period, we included 30 cases of staphylin dermatitis among 10239 consulting patients, representing a hospital frequency of 0.3%.
Males accounted for 63% of cases (19/30), representing a sex ratio of 1.7.
The mean age was 13 years, ranging from 1 to 64 years. The 0-14 age group accounted for 57% of cases (17/30), the 15-24 age group for 10% (3/30), and the 25-64 age group for 33% (10/30). Patients living in the city accounted for 94% of cases (28/30). The functional signs were pruritus in 47% of cases, burning in 43% and both signs were associated in 10% of cases. Clinically, erythema was present in 47% of cases, bullae in 43%, vesicles in 30%, pustules in 17%, crusts in 10%, and erosions and infiltrations in 3% each (Table 1).
| Table 1: Dermatological Profile of Staphylin Dermatitis Cases. | |||||||
| N° | Initials | Age (year) | SEX | Clinical Description | Headquaters | Traitment | Evolution |
| 1 | HS | 12 | M | Bubble | Neck, Trunk | Antiseptic and dermocorticoid | < 10 J |
| 2 | BK | 6 | M | Érythema, Pustules | Neck, Thigh | Antiseptic and zinc oxyde, dermocorticoïd | < 10 J |
| 3 | KK | 2 | M | Vésicles, Bubble | Oculoconjunctival | Zinc oxyde and Fucloxacillin | < 10 J |
| 4 | SC | 18 | F | Bubbles and Pustules | Face and Arms | Antiseptic, | < 10 j |
| 5 | DD | 5 | M | Pustules | Face | Zinc oxyde | < 10 J |
| 6 | SK | 31 | F | Érythema and crusts | Face and Neck | Antiseptic and Zinc oxyde | < 10 J |
| 7 | FC | 32 | F | Crusts | Arms and trunk | Fucidine cream and antiseptic | < 10 j |
| 8 | KK | 6 | M | Érythema | Face | Antiseptic and zinc oxyde | < 1O J |
| 9 | AC | 22 | F | Érythema | Neck | Zinc oxyde | < 10 J |
| 10 | AC | 13 | F | Érythema and Vésicles | Face and trunk | Antiseptic and zinc oxyde | < 10 J |
| 11 | AB | 1 | M | Bubbles | Thigh | Zinc oxyde | < 10 J |
| 12 | AC | 13 | F | Bubbles and vésicles | Back and Face | Antiseptic and zinc oxyde | < 10 J |
| 13 | LT | 62 | M | Bubbles and Crusts | Neck | Antiseptic and zinc oxyde | < 10 J |
| 14 | DO | 37 | F | Vésicles and pustules | Trunk and arms | Antiseptic, antibiotic and zinc oxyde | < 10 J |
| 15 | MK | 3 | M | Bubbles | Face | Antiseptic, antibiotic and zinc oxyde | < 10 J |
| 16 | HC | 14 | M | Érythema and edema | Oculoconjunctival | Dermocorticoïd | < 10 J |
| 17 | AC | 30 | F | Vesicles | Neck | Zinc oxyde | < 10 J |
| 18 | MD | 64 | M | Vésicles | Trunk | Zinc Oxyde, dermocorticoïd | < 10 J |
| 19 | DC | 26 | M | Érythema and bubbles | Face | Zinc oxyde and antiseptic and antibiotic | < 10 j |
| 20 | MC | 12 | F | Bubbles and Pustules | Neck | Antiseptic, zinc oxyde and, dermocorticoïd | < 10 j |
| 21 | MF | 2 | M | Vésicles | Neck | Antiseptic, Zinc oxyde | < 10 J |
| 22 | BB | 7 | M | Bubbles | Neck | Antiseptic, zin oxyde | < 10 j |
| 23 | AS | 9 | M | Érosion | Pédicular | Antiseptic and antibiotic | < 10 J |
| 24 | AS | 19 | M | Bubbles | Neck | Antiseptic and zinc oxyde | < 10 J |
| 25 | FK | 3 | M | Érythema and vesicles | Face | Zinc oxyde | < 10 J |
| 26 | MD | 35 | F | Bubbles | Face | Antiseptic | < 10 J |
| 27 | ST | 39 | M | Érythema | Thigh | Antiseptic and zinc oxyde | < 10 J |
| 28 | LC | 34 | F | Bubbles and Pustules | Arms and trunk | Antiseptic and zinc oxyde | < 10 J |
| 29 | KK | 8 | M | Érythema | Face | Zinc oxyde | < 10 J |
| 30 | LM | 25 | M | Érythema and Vésicles | Neck | Antiseptic and zinc oxyde | < 10 J |
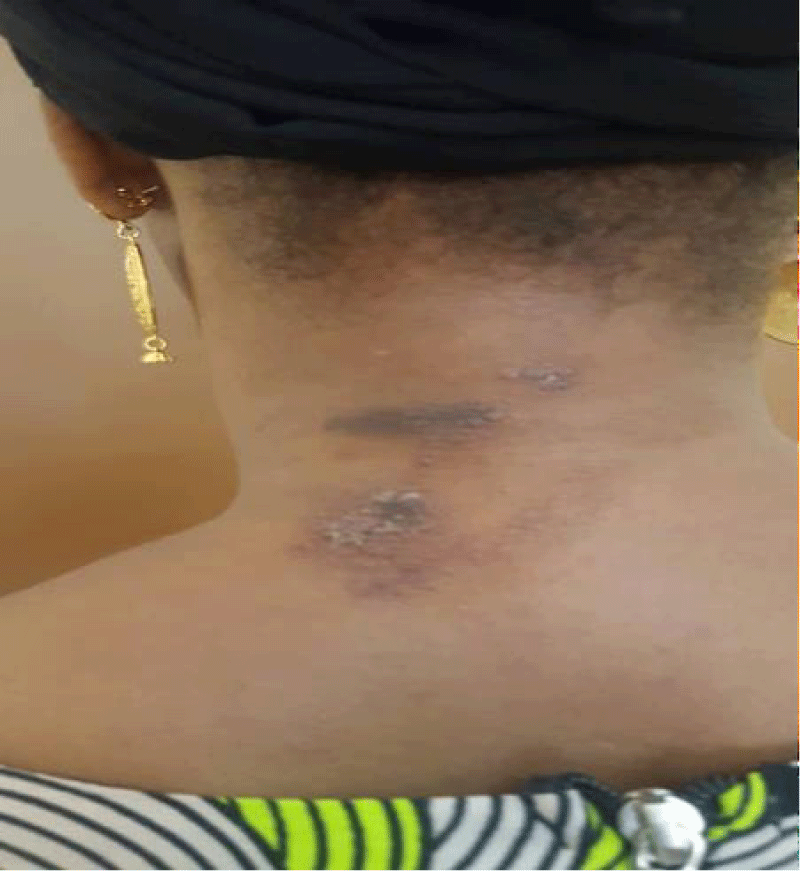
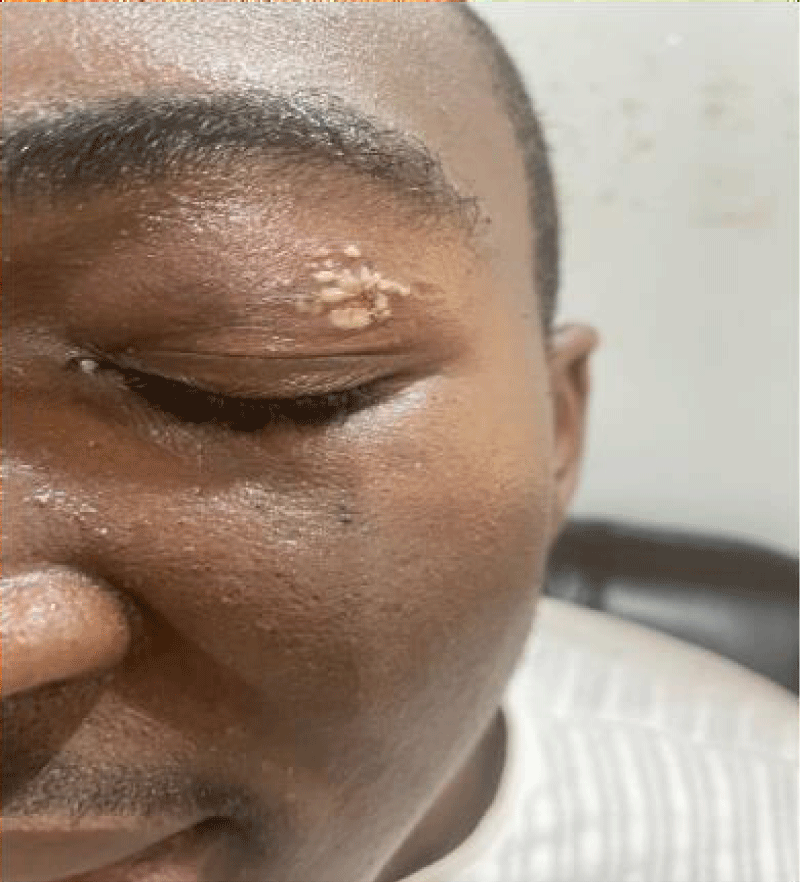
According to topography, the head was affected in 40% of cases, including the face in 23%, and the area around the eyes in 17% of cases. The neck was affected in 33% of cases, and the trunk and limbs in 10% (Figures 1,2). Involvement of the neck and face was reported in one case, and involvement of the arm and face in another.
Figure 1: Neck damage.
Figure 2: Periocular damage.
We described the epidemic-clinical aspects of cases of staphylin dermatitis at the Dermatology Hospital in Bamako. We recruited cases during the period October - December 2022, corresponding to the end of the rainy season, a period usually conducive to insect proliferation [2].
The diagnosis of staphylin dermatitis was anamnestic and clinical. It was based on the notion of the occurrence of an acute, non-systematized erosive dermatitis, accompanied by functional signs such as burning, and localized preferentially on the uncovered parts of the skin.
The limitations of this study were its strictly hospital-based recruitment at the Bamako Dermatology Hospital and the limited number of cases. In fact, beetles have mainly been described in the Sikasso region, where rainfall is higher [2]. However, this work has enabled us to describe the epidemioclinical aspects of staphylin dermatitis at the HDB.
Discussion of the data
The hospital frequency reported in our work may be underestimated, due to the benign and acute nature of this condition, and also to the fact that a large number of cases are managed at home. This limits the number of cases coming to the hospital for consultation. Some patients were able to consult peripheral centers. Some complicated forms may have been labeled as impetigo and not counted.
In Africa, the number of cases varies from study to study. The authors describe cases occurring between October and December [2,4,5]. These are periods of heavy rainfall during which insect proliferation has been described [6].
These eco-climatic factors have been widely reported. Authors describe cases in particular climatic zones; in India, Gnanaraj P describes cases in a hot zone characterized by a long summer. In Brazil, Fonseca JMV reports 19 cases in a town near a river. Guinea and Cameroon are renowned for their hot, humid climate [7,8]. In Iran, Zargari O also described cases in summer during periods of heavy rainfall. Some authors have described cases in the context of epidemics [5] or in expatriate subjects [3]. Vanhecke [3] reports 74 cases over a 3-month period in expatriate subjects in Guinea and 19 cases in native populations in Cameroon. The higher number of cases reported in Guinea [3] compared with our series and that of Cameroon [9,10] may be explained by better notification of cases. In fact, in Guinea, these were expatriate patients who consulted a dedicated health center. Some authors have reported cases in rural or forested areas, where insects are frequently found [11,12].
Our study took place in Bamako, the capital of Mali, and this predominance of urban cases was explained by the attraction of insects to light [13]. Contact with the insect is most often in the evening.
In fact, in Malaysia [12], cases have been described in children attending evening classes. According to some authors, pederine is produced mainly by female coleoptera, which produce the substance in the evening. A pseudomonas bacterium is thought to be responsible for the production of this substance [14].
Our series was predominantly male. These data are in line with those from Cameroon [8]. In Guinea, authors [3,15] reported parity or female predominance. Gender predominance in the various studies may be fortuitous. In our series, children were the most represented. In Cameroon and Guinea [3,8,15], adults predominated. The disparities between these studies can be explained by their different methodological approaches. In our series, the patients were recruited from urban hospitals. In the other studies, recruitment took place in a specific context, a given village [8], or a specialized center for expatriates [3,15]. In Malaysia, the study focused solely on schoolchildren. Pruritus was the most frequently described functional sign in our cases. In Malaysia, pruritus was also the predominant functional sign among cases reported in children [12]. In Guinea [3,8,15], pain is described as burning. However, these are functional signs that depend on the patient's feelings.
The most frequent elementary lesions were erythema and bullae. These clinical aspects were described by all African authors. Vanhecke C, Tounkara TM, The predominance of erythema and bullae expresses the acute nature of the disease in our cases. Lesions were mainly located on the face in our cases. In Guinea, Tounkara TM describes a predominance of lesions on the neck. As a general rule, all authors describe a predominance of lesions on the neck and face [3,5,15]. These are exposed areas not covered by clothing and accessible to flying insects.
- Uzunoğlu E, Oguz ID, Kir B, Akdemir C. Clinical and Epidemiological Features of Paederus Dermatitis Among Nut Farm Workers in Turkey. Am J Trop Med Hyg. 2017 Feb 8;96(2):483-487. doi: 10.4269/ajtmh.16-0582. Epub 2016 Nov 22. PMID: 27879459; PMCID: PMC5303057.
- Koné and al Rural Economy Institute (IER), Regional Center for Agronomic Research (CRRA) Sikasso-Mali. Laboratoire dentomologie and Parasitologie (LEP), Faculty of Science and Technology (FST), University of Sciences, Techniques and Technologies of Bamako (USTTB), Bamako, Mali.
- Vanhecke C, Malvy D, Guevart E, Laloge V, Ezzedine K. Dermatite àPaederus : étude rétrospective de 74 cas survenus en 2008 à Conakry, Guinée [Paederus dermatitis: A retrospective study of 74 cases occurring in 2008 in Guinea-Conakry]. Ann Dermatol Venereol. 2010 Mar;137(3):189-93. French. doi: 10.1016/j.annder.2010.02.003. Epub 2010 Feb 24. PMID: 20227560.
- Van Schayk IMCJ, Agwanda RO, Githure JI, Beier JC, Knols BGJ. El Niño causes dramatic outbreak of Paederus dermatitis in East Africa. In: Low PS, éditeur. Climate Change and Africa. 1re éd. Cambridge University Press; 2005. 240‑7. https://www.cambridge.org/core/product/identifier/CBO9780511535864A044/type/book_part
- Alva-Davalos V, Laguna-Torres VA, Huaman A, Olivos R, Chavez M, Garcia C, Mendoza N. Dermatite epidêmica por Paederus irritans em Piura, Perú, 1999, relacionada ao fenômeno El Niño [Epidemic dermatits by Paederus irritans in Piura, Perú at 1999, related to El Niño phenomenon]. Rev Soc Bras Med Trop. 2002 Jan-Feb;35(1):23-8. Portuguese. doi: 10.1590/s0037-86822002000100005. PMID: 11873257.
- Fonseca JMV, Oliveira CMND, Peluzio RJE, Zanúncio JC, Fiorezi JMS. Vesicant dermatitis caused by Paederus sp.: report of 19 cases in Viçosa, Minas Gerais, Brazil. Rev Bras Med Fam E Comunidade. 2012; 7(25): 255-8.
- Gnanaraj P, Venugopal V, Mozhi MK, Pandurangan CN. An outbreak of Paederus dermatitis in a suburban hospital in South India: a report of 123 cases and review of literature. J Am Acad Dermatol. 2007 Aug;57(2):297-300. doi: 10.1016/j.jaad.2006.10.982. Epub 2007 May 9. PMID: 17490784.
- Vanhecke C, Le Gall P, Gaüzère BA. Dermatite vésicante à Paederus au Cameroun et revue de littérature [Vesicular contact dermatitis due to Paederus in Cameroon and review of the literature]. Bull Soc Pathol Exot. 2015 Dec;108(5):328-36. French. doi: 10.1007/s13149-015-0459-9. Epub 2015 Nov 25. PMID: 26608274.
- Zargari O, Kimyai-Asadi A, Fathalikhani F, Panahi M. Paederus dermatitis in northern Iran: a report of 156 cases. Int J Dermatol. 2003 Aug;42(8):608-12. doi: 10.1046/j.1365-4362.2003.01771.x. PMID: 12890103.
- Vanhecke C, Le Gall P, Gaüzère BA. Dermatite vésicante à Paederus au Cameroun et revue de littérature [Vesicular contact dermatitis due to Paederus in Cameroon and review of the literature]. Bull Soc Pathol Exot. 2015 Dec;108(5):328-36. French. doi: 10.1007/s13149-015-0459-9. Epub 2015 Nov 25. PMID: 26608274.
- Mammino JJ. Paederus dermatitis: an outbreak on a medical mission boat in the Amazon. J Clin Aesthet Dermatol. 2011 Nov;4(11):44-6. PMID: 22125660; PMCID: PMC3225135.
- Rahmah E, Norjaiza MJ. An outbreak of Paederus dermatitis in a primary school, Terengganu, Malaysia. Malays J Pathol. 2008 Jun;30(1):53-6. PMID: 19108412.
- Kamaladasa SD, Perera WD, Weeratunge L. An outbreak of paederus dermatitis in a suburban hospital in Sri Lanka. Int J Dermatol. 1997 Jan;36(1):34-6. doi: 10.1046/j.1365-4362.1997.00009.x. PMID: 9071612.
- Piel J. A polyketide synthase-peptide synthetase gene cluster from an uncultured bacterial symbiont of Paederus beetles. Proc Natl Acad Sci U S A. 2002 Oct 29;99(22):14002-7. doi: 10.1073/pnas.222481399. Epub 2002 Oct 14. PMID: 12381784; PMCID: PMC137826.
- Tounkara TM, Diane BF, Keita M, Soumah MM, Kante MD, Keita F. Paederus dermatitis: Clinical and epidemiological characteristics of 104 new cases collected at the University Hospital of Conakry, Guinea. Our Dermatol Online. 2022; 13(Supp 2):27‑33.