More Information
Submitted: July 24, 2023 | Approved: August 04, 2023 | Published: August 07, 2023
How to cite this article: Belda W, Jr, Passero LF. Rare Presentation of Chromoblastomycosis Due to Multiple Simultaneous Inoculations: A Case Report. Ann Dermatol Res. 2023; 7: 032-035.
DOI: 10.29328/journal.adr.1001028
Copyright License: © 2023 Belda W, Jr, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Chromoblastomycosis, Treatment, Rare Presentation, Multiple Inoculations
Abbreviations: CBM:: Chromoblastomycosis
Rare Presentation of Chromoblastomycosis Due to Multiple Simultaneous Inoculations: A Case Report
Walter Belda Jr1* and Luiz Felipe Passero2
1Dermatology Department, University of São Paulo, Medical School (USP) ,São Paulo, Brazil
2Institute of Biosciencies, São Paulo State University (UNESP), Sao Vicente, Brazil
*Address for Correspondence: Walter Belda Jr, Dermatology Department, University of São Paulo. Av. Açocê, 162, Moema, São Paulo, CEP=04075-020, Brazil, Email: [email protected]
Chronic infectious, granulomatous and suppurative dermatosis, classified among the subcutaneous mycoses, prevalent in tropical and subtropical regions and caused by the traumatic implantation of dematiaceous fungal species, where the presence of muriform bodies is an expression of the causal agent in the grafted tissue, are characteristic of chromoblastomycosis. Considered the second implantation mycosis in the world, it manifests itself with slow and progressive growth lesions of exophytic and verrucous plaques and black dots on the surface.
The disease is considered a neglected and occupational disease, which occurs mainly among agricultural workers, coconut and babassu harvesters, lumberjacks, and traders of agricultural products. It is important to highlight that people at risk of contracting chromoblastomycosis work in tropical countries, where the temperature can be above 40 °C in summer, and generally refuse to wear protective equipment during the day (shoes, gloves, clothes, etc.), although they know that this type of prophylactic measure can prevent different types of diseases. These vulnerable people often live in low-income countries and sometimes live far from medical services and, once infected, do not seek medical attention. We report below an exuberant and unusual case due to multiple simultaneous inoculations on the back after the patient suffered an accidental fall while working on his farm. The diagnosis was established by direct mycological examination, histopathological examination, culture, microculture, and MALDI-TOF MS analysis that identified the agent of the Fonsecaea pedrosoi.
Chromoblastomycosis is a chronic infectious, granulo-matous, and suppurative dermatosis, classified among sub-cutaneous mycoses, caused by pigmented dematiaceous fungi species, more prevalent in tropical and subtropical regions. The manifestation of chromoblastomycosis occurs after the traumatic implantation of dematiaceous fungal species in the skin, and the presence of muriform bodies is an expression of the etiologic agent in the tissue. Chromo-blastomycosis is considered the second implantation mycosis in the world, where skin lesions grow slowly as progressive exophytic and verrucous plaques and black dots on the surface [1].
The disease is considered an occupational disease, which occurs mainly in agriculture, whose main activity is related to conventional methods to grow and harvest plants, making it easier for humans to come into contact with etiological agents. In addition, such individuals sometimes work without protection, such as gloves, shoes, or clothing, which can prevent skin damage and subsequent infection [2].
After accidental implantation, a small lesion appears, but patients generally do not notice any inoculation. It initially presents as an isolated macular lesion, progressing to a papular lesion that gradually increases in size over a few weeks. It evolves into a papulosquamous form, sometimes with a polymorphic appearance, which can be confused with several other types of skin infections. The lesions gradually assume a verrucous aspect; however, in the beginning, the lesions are asymptomatic and usually do not interfere with the patient's activities. Over time, patients may experience moderate pruritus, but it can be intense and accompanied by local pain [3]. It progresses in extension along the skin but does not directly affect the deep tissues.
The slow advance in the skin due to contiguity produces fibrotic changes and lymphatic stasis, leading to lymphedema, which in some cases resembles elephantiasis. Secondary recurrent bacterial infection is another common complication. This process exacerbates lymphatic vessel involvement [1]. When it begins in the lower extremities, the picture tends to expand to the knee, thigh, or dorsum of the foot, sparing the plantar region. The occurrence of unusual or atypical clinical presentations is very rarely reported, which in itself can hinder or delay the diagnosis and adequate therapy [4,5].
In the present manuscript, we present a rare and unusual case of the appearance of verrucous lesions clinically compatible with chromoblastomycosis, on the back of a patient after he suffered a fall while working on the farm, which caused several abrasions, leading to the simultaneous appearance of these lesions, removing the possibility that hematogenous or lymphatic spread has occurred from an initial lesion.
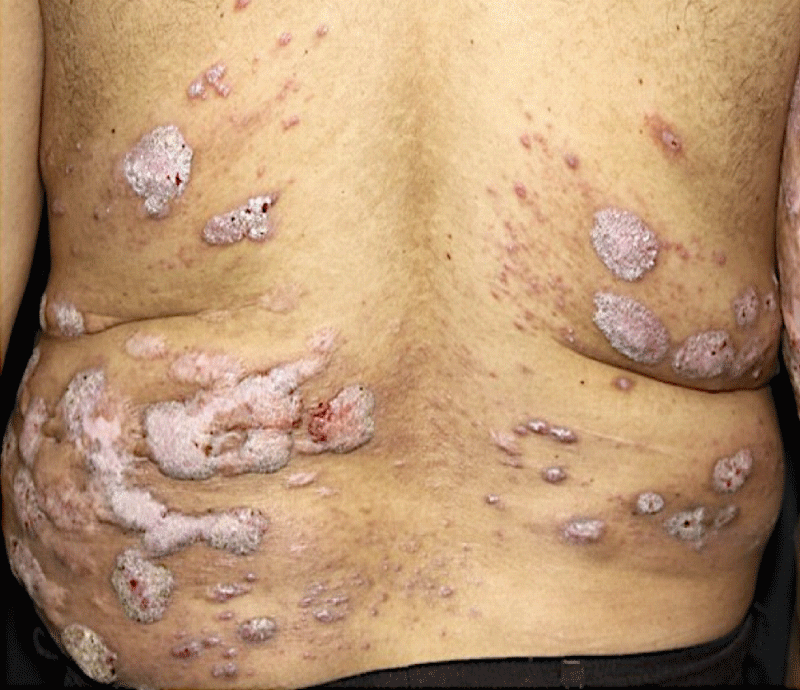
A 49-year-old male patient, farmer and resident of Santa Barbara, Bahia, Brazil reported the appearance of verrucous lesions simultaneously in the back, initially small, without symptoms, which gradually increased and affected several areas of the back. Six years before the appearance of the injury, he was working in the field, wearing only long pants and boots, but not a shirt. At that moment, he suffered a fall on the land and bruised his back in several places. Superficial lesions healed, but others increased in size and looked like small warts, some of them with purulent discharge and a slightly fetid odor. After a few months of its appearance, he sought medical attention, being medicated with local and systemic antibiotics, with no result. With little improvement in the condition, he abandoned medical follow-up. He came to the Department of Dermatology of Medical School of the São Paulo University six years after the onset of the condition with multiple verrucous lesions of varying shapes and sizes (Figure 1), which affected a large area of the back and posterior face of the right arm. He reported local discomfort and sometimes a fetid odor emanating from the lesions. Blood biochemical levels of glycemia, cholesterol, liver transaminases, gamma-glutamyl transferase, triglycerides, bilirubin, urea, and creatinine were normal; additionally, the patient was serologically negative for hepatitis, syphilis and HIV.
Figure 1: Multiple verrucous lesions of different sizes affecting the patient's back.
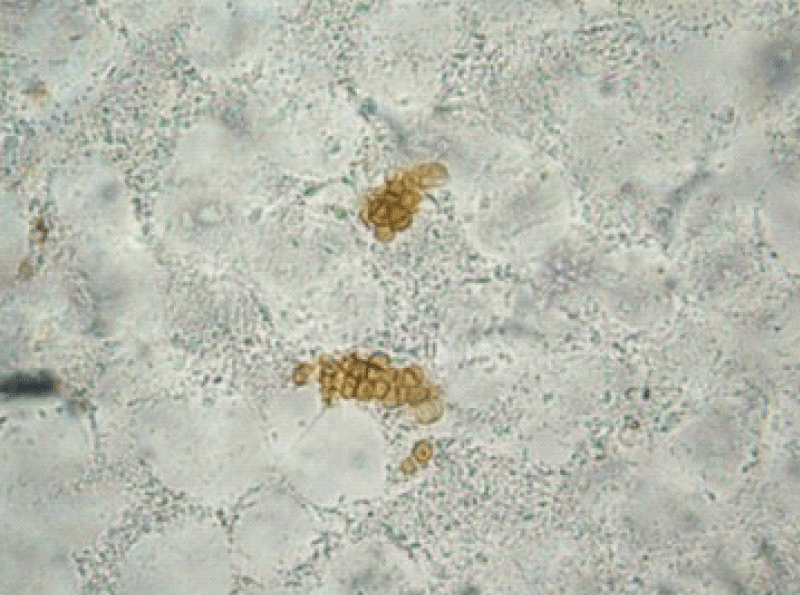
Microscopic examination of the three different lesions in the same time scales in a 10% potassium hydroxide (KOH) wet mount revealed the presence of muriform cells in all samples (Figure 2).
Figure 2: Direct mycological examination of the verrucous lesion demonstrating muriform bodies.
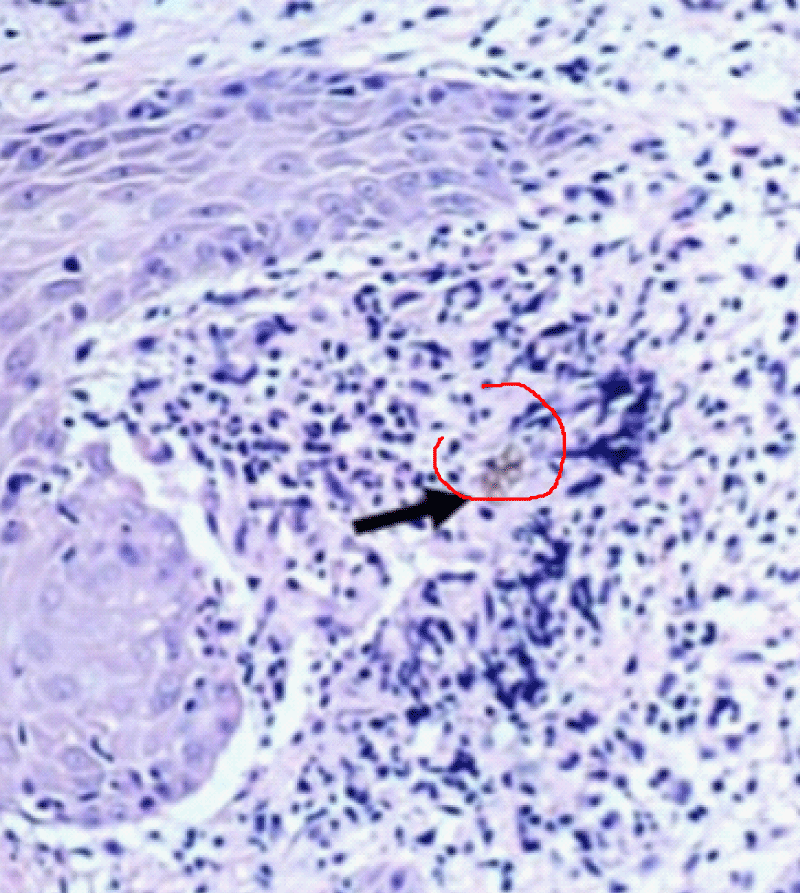
The histopathological study performed on skin sections, collected from his back showed pseudo-epitheliomatous hyperplasia in the epidermis and muriform cells surrounded by granuloma in the dermis (Figure 3).
Figure 3: Histopathological examination demonstrating muriform bodies in the tissue.
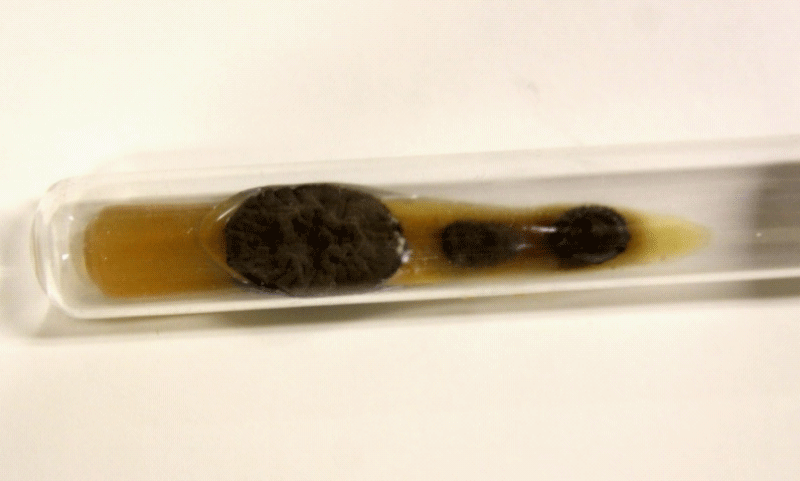
Figure 4: Black aspect of culture on Agar sabouraud medium.
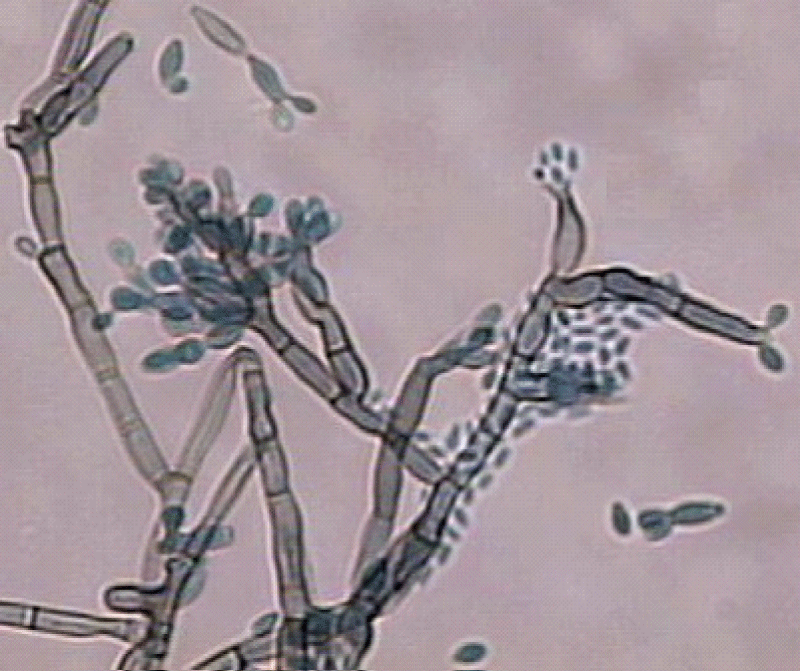
Figure 5: Microculture aspect.
Colonies of the fungus were subjected to MALDI-TOF MS analysis to identify species of the etiologic agent. Standard protein extraction was performed using ethanol and formic acid [6] and 1μL aliquot of the sample was submitted to MALDI-TOF MS analysis using the Vitek MS™ instrument (bioMérieux). For each acquisition group, a standard (Escherichia coli ATCC 8739) was included to calibrate the instrument and validate the run. Spectra were generated using LaunchpadTM v2.8 software (bioMérieux) and analyzed using SARAMIS Premium v4.11TM for Research Use Only (RUO) software SARAMIS Premium v4.11™ (bioMérieux).
All biological material seeded on Sabouraud Agar at 25 oC was positive for Fonsecae sp. Furthermore, MALD-TOF MS analysis allowed us to correctly identify the species Fonsecae pedrosoi as the etiologic agent with 99.9% confidence level values by comparing the obtained spectra with a new in-house SuperSpectrum library HCFMUSP 02 [7].
Blood biochemical levels of glycemia, cholesterol, liver transaminases, gamma-glutamyl transferase, triglycerides, bilirubin, urea, and creatinine were normal; additionally, the patient was serologically negative for hepatitis, syphilis, and HIV.
Since the description of the first case of CBM, different clinical forms have been described and a variety of pathogenic fungal species have been incriminated as etiologic agents of this disease. In general, CBM agents live in organic material in soil, plants, and aquatic environments; therefore, in addition to being considered a new disease, there is a complex epidemiological scenario associated with CBM.
Several species of fungus can cause CBM, in Brazil, the Fonsecaea pedrosoi complex predominates, but Phialophora verrucosa, Cladophialophora carrionii, Rhinocladiella aquaspersa and Exophiala spinifera, Cladophialophora arxii, Wangiella dermatitidis, and Botryomyces caespitosus are also recognized as possible agents [8,9]. The period of incubation of CBM is uncertain, but the evolution of the disease is slow and progressive, with the lower extremities being the most commonly affected area of the body. Hands, arms and buttocks are also often affected, and sporadic reports mention injuries to the ears, face, breasts, and armpits. In the beginning, the disease remains localized to the area of the initial injury or the nearby skin, but lymphatic and hematogenous spread may occur, producing metastatic lesions.
The main differential diagnosis of CBM should be made with other fungal diseases such as lobomycosis, sporotrichosis, protothecosis, paracoccidioidomycosis, eumycetoma, phaeohyphomycosis, granulomatous derma-tophytosis, and Majochi's granuloma; furthermore, it is important to differently diagnose some bacterial diseases such as verrucous tuberculosis, leprosy, actinomycetoma, nocardiosis, botryomycosis, tertiary syphilis, and atypical mycobacteriosis. A differential diagnosis must also be made with cutaneous leishmaniasis which is a parasitic disease, as well as with non-infectious diseases such as squamous cell carcinoma, keratoacanthoma, lupus erythematosus, cutaneous sarcoidosis, and mycosis fungoides. The clinical appearance of the lesions, location, clinical history, and evolution can help to limit the differential diagnosis [10,11].
In the treatment of this pathology except for small initial lesions that can be cured by surgical removal, CBM lesions constitute a real therapeutic challenge for physicians and patients [12].
There are no publications in the literature of randomized or comparative clinical and therapeutic trials that can show which is the best treatment. To achieve therapeutic success, one must consider the patient's health status, socioeconomic conditions, the possibility of adherence to the proposed therapy, and the presence of comorbidities [13,14].
In recent years, there have been several reports of small case series evaluating the combination of antifungal drugs such as itraconazole or terbinafine with immunomodulatory compounds such as glucan [15] and topical imiquimod [16], as well as the use of imiquimod as monotherapy [17] with promising results.
Acitrein, in association with itraconazole, is another drug recently used as an adjuvant therapy, particularly in more extensive cases, which has shown great promise in rapidly reducing the appearance of verrucous, facilitating and reducing treatment time for this pathology [18].
In the present case, therapy was introduced with itraconazole 200 mg/day associated with acitretin 50 mg/day and topical application of imiquimod. The patient remains under outpatient follow-up.
In this manuscript, we report the manifestation of CBM on the back of a patient, in fact, an unusual presentation, whose lesions began to develop simultaneously after he suffered an accidental fall during his fieldwork, which led to the development of several abrasions. Some of these healed, but others began to develop lesions that developed a verrucous aspect, thus ruling out the hypothesis of the occurrence of lesions by lymphatic or hematogenous dissemination to sites close to the initial lesion and consequently, the lesions began to appear in several places simultaneously, demonstrating the implantation of the fungus in several places on the patient's back at the same time.
In the event of lesions with a verrucous aspect that has evolved for a long time in any region of the skin of a patient, the doctor must obligatorily make the differential diagnosis with the main diseases that can assume this clinical aspect, mainly with squamous cell carcinoma, particularly when located in photo exposed areas, as in the present case. As with other deep mycoses common in rural areas, such as verrucous paracoccidioidomycosis and sporotrichosis, in addition to cases of verrucous tuberculosis. The relevant fact in this case is the simultaneous appearance of several lesions with a verrucous appearance on the patient's back, which could initially confuse the attendant, since this is not the classic aspect of CBM, thus delaying the start of the specific treatment. It should be noted that a thorough clinical examination of the lesions is necessary and, in the case of suspected chromoblastomycosis, the presence of black dots on the surface makes this diagnosis very suggestive.
- Queiroz-Telles F. CHROMOBLASTOMYCOSIS: A NEGLECTED TROPICAL DISEASE. Rev Inst Med Trop Sao Paulo. 2015 Sep;57 Suppl 19(Suppl 19):46-50. doi: 10.1590/S0036-46652015000700009. PMID: 26465369; PMCID: PMC4711190.
- Guevara A, Siqueira NP, Nery AF, Cavalcante LRDS, Hagen F, Hahn RC. Chromoblastomycosis in Latin America and the Caribbean: Epidemiology over the past 50 years. Med Mycol. 2021 Dec 8;60(1):myab062. doi: 10.1093/mmy/myab062. PMID: 34637525.
- López Martínez R, Méndez Tovar LJ. Chromoblastomycosis. Clin Dermatol. 2007 Mar-Apr;25(2):188-94. doi: 10.1016/j.clindermatol.2006.05.007. PMID: 17350498.
- Tele J, Gupta S, Patil N. Cutaneous chromoblastomycosis-unusual presentation: a case report. Int J Res Med Sci, 2018;6:2856-2859
- Belda W, Carvalho CHC, Passero LFD. Chromoblastomycosis with 18 years of evolution. Int J Res Med Sci. 2023 Aug;11(8):3034-3038
- de Almeida Júnior JN, Figueiredo DS, Toubas D, Del Negro GM, Motta AL, Rossi F, Guitard J, Morio F, Bailly E, Angoulvant A, Mazier D, Benard G, Hennequin C. Usefulness of matrix-assisted laser desorption ionisation-time-of-flight mass spectrometry for identifying clinical Trichosporon isolates. Clin Microbiol Infect. 2014 Aug;20(8):784-90. doi: 10.1111/1469-0691.12502. Epub 2014 Jan 16. PMID: 24355037.
- de Almeida JN Jr, Favero Gimenes VM, Francisco EC, Machado Siqueira LP, Gonçalves de Almeida RK, Guitard J, Hennequin C, Colombo AL, Benard G, Rossi F. Evaluating and Improving Vitek MS for Identification of Clinically Relevant Species of Trichosporon and the Closely Related Genera Cutaneotrichosporon and Apiotrichum. J Clin Microbiol. 2017 Aug;55(8):2439-2444. doi: 10.1128/JCM.00461-17. Epub 2017 May 24. PMID: 28539340; PMCID: PMC5527422.
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018 Jul-Aug;93(4):495-506. doi: 10.1590/abd1806-4841.20187321. PMID: 30066754; PMCID: PMC6063100.
- de Andrade TS, de Almeida AMZ, Basano SA, Takagi EH, Szeszs MW, Melhem MSC, Albuquerque M, Camargo JSAA, Gambale W, Camargo LMA. Chromoblastomycosis in the Amazon region, Brazil, caused by Fonsecaea pedrosoi, Fonsecaea nubica, and Rhinocladiella similis: Clinicopathology, susceptibility, and molecular identification. Med Mycol. 2020 Feb 1;58(2):172-180. doi: 10.1093/mmy/myz034. PMID: 31329924.
- Schwalb A, Seas C. Chromoblastomycosis. N Engl J Med. 2020 Jul 9;383(2):e7. doi: 10.1056/NEJMicm1913199. PMID: 32640136.
- Baka JLCES, Giraldelli G, Bernardes-Engemann AR, Barcaui CB, Orofino-Costa R. Urban chromoblastomycosis: a diagnosis that should not be neglected. An Bras Dermatol. 2023 May-Jun;98(3):422-425. doi: 10.1016/j.abd.2022.01.013. Epub 2023 Feb 4. PMID: 36746731; PMCID: PMC10173060.
- Castro LG, Andrade TS. Chromoblastomycosis: still a therapeutic challenge. Expert Rev Dermatol. 2010; 5:433-443.
- Martinez RL, Tovar LJ. Cromoblastomycosis. Clin Dermatol. 2007; 25:188-194.
- Seas C, Legua P. Mycetoma, chromoblastomycosis and other deep fungal infections: diagnostic and treatment approach. Curr Opin Infect Dis. 2022 Oct 1;35(5):379-383. doi: 10.1097/QCO.0000000000000870. Epub 2022 Aug 3. PMID: 35942857.
- Azevedo Cde M, Marques SG, Resende MA, Gonçalves AG, Santos DV, da Silva RR, de Sousa Mda G, de Almeida SR. The use of glucan as immunostimulant in the treatment of a severe case of chromoblastomycosis. Mycoses. 2008 Jul;51(4):341-4. doi: 10.1111/j.1439-0507.2007.01485.x. Epub 2008 Apr 28. PMID: 18444974.
- de Sousa Mda G, Belda W Jr, Spina R, Lota PR, Valente NS, Brown GD, Criado PR, Benard G. Topical application of imiquimod as a treatment for chromoblastomycosis. Clin Infect Dis. 2014 Jun;58(12):1734-7. doi: 10.1093/cid/ciu168. Epub 2014 Mar 14. PMID: 24633683; PMCID: PMC4036686.
- Belda W Jr, Criado PR, Passero LFD. Successful treatment of chromoblastomycosis caused by Fonsecaea pedrosoi using imiquimod. J Dermatol. 2020 Apr;47(4):409-412. doi: 10.1111/1346-8138.15225. Epub 2020 Jan 21. PMID: 31960479.
- Belda W, Criado PR, Domingues Passero LF. Case Report: Treatment of Chromoblastomycosis with Combinations including Acitretin: A Report of Two Cases. Am J Trop Med Hyg. 2020 Nov;103(5):1852-1854. doi: 10.4269/ajtmh.20-0471. PMID: 32815507; PMCID: PMC7646813.