More Information
Submitted: June 14, 2023 | Approved: July 01, 2023 | Published: July 03, 2023
How to cite this article: Dehkordi RAF, Pasalar S, Dehkordi SH, Karimi B. The Combinatory Effects of Zinc Oxide Nanoparticles (ZnO NPs) and Thiamine on Skin of Alloxan-Induced Diabetic Mice; a Stereological and Biochemical Study. Ann Dermatol Res. 2023; 7: 018-027.
DOI: 10.29328/journal.adr.1001026
Copyright License: © 2023 Dehkordi RAF, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Diabetes; Mice; Skin; Thiamine; ZnO NPs
Abbreviations: GGT: Gamma-Glutamyl Transferase; BUN: Blood Urea Nitrogen; Cr: Creatinine; MDA: Malondialdehyde; ZnO NPs: Zinc Oxide Nanoparticles; ZnO: Zinc Oxide; COA: Acetylsalicylic Acid; NADPH: Nicotinamide Adenine Dinucleotide Phosphate; IP: Intra-Peritoneal; H&E: Hematoxylin and Eosin; TBA: Thiobarbituric Acid.
The Combinatory Effects of Zinc Oxide Nanoparticles (ZnO NPs) and Thiamine on Skin of Alloxan-Induced Diabetic Mice; a Stereological and Biochemical Study
Rahmat Allah Fatahian Dehkordi1*, Sekineh Pasalar2, Saied Habibian Dehkordi3 and Bahnaz Karimi4
1Associate Professor, Department of Basic Sciences, Veterinary Anatomy and Histology Section, Faculty of Veterinary Medicine, Shahrekord University, Shahrekord, Iran
2Master Student of Histology, Faculty of Veterinary Medicine, Shahrekord University, Shahrekord, Iran
3Associate Professor, Department of Pharmacology, School of Veterinary Medicine, Shahrekord University, Shahrekord, Iran
4Assisstance Professor, Department of Basic Sciences, Veterinary Biochemistry Section, Faculty of Veterinary Medicine, Shahrekord University, Shahrekord, Iran
*Address for Correspondence: Rahmat-Allah Fatahian Dehkordi, Associate Professor, Department of Basic Sciences, Veterinary Anatomy and Histology Section, Faculty of Veterinary Medicine, Shahrekord University, Shahrekord, Iran, Email: [email protected]
This study aimed to investigate the treatment effects of ZnO NPs plus thiamine on histo-stereological and biochemical parameters in diabetic mouse skin. In total 54 BALB/C mice were used and divided into nine groups. Twenty-four mice were coordinately assigned as control groups (I), thiamine (II), and zinc oxide nanoparticles (III and IV; 1.0 and 5.0 mg/kg). Diabetes was induced in the remaining rats with a dose of 180 mg/kg of alloxan; diabetes group (group V). Other diabetic mice were treated with ZnO NPs (0.1 and 0.5 mg/kg) alone (groups VI and VII, respectively) and along with thiamine (groups VIII and IX, respectively). An increase in weight was observed in the diabetic group compared to the control group. Diabetic skin showed decreasing in volume density of collagen bundles and decreasing in the epidermis and dermis thickness, as well as an increase in the hypodermis's thickness. Administration of ZnO NPs (0.1 and 0.5 mg/kg) alone and along with thiamine in the diabetic animals resulted in anti-hyperglycemic activity, reducing GGT, BUN, Cr, MDA, and NO levels in treated diabetic mice. In conclusion, the concomitant use of ZnO NPs along with thiamine presents the potential as a combination therapy for the treatment of alloxan-induced diabetic mice skin changes.
Diabetes is a chronic metabolic disease in which some disturbances occur in the metabolism of lipids, proteins, and carbohydrates, besides an increase in blood glucose level [1-3]. Complications followed by this disease include nephropathy, retinopathy, and neuropathy. It can trigger cardiovascular disorders like cardiac infarction and hypertension and also cataracts in the eyes [4]. Since some researchers have found changes in the skin epidermis in diabetic patients, this has led to increased attention to the study of non-injured diabetic skin [5]. Skin diseases caused by diabetes have serious side effects; therefore, recognizing the most effective treatment measures results from a better understanding of the pathology of diabetic skin disorders [6]. Although several factors may be involved in chronic skin complications in diabetics, these side effects become more complicated because of changes in the skin's structure. Besides, fibroblast growth failure decreased collagen synthesis, and, in particular, accumulation of advanced glycation end products (AGEs) occurs in diabetic skin complications [7].
In recent years, many studies have been conducted on zinc oxide (ZnO) and various forms of its nanostructures. It has also a vast application in sensors due to its high sensitivity to chemicals and high contact surface. ZnO NPs trap light due to their high contact surface [8], therefore, these nanoparticles are frequently used in the manufacture of solar cells. Other applications of these compounds are iron galvanization [9], pigments, corrosion-preventing agents, production of toothpaste, sunscreens, etc. [10]. ZnO NPs have antibacterial and antifungal properties that are used in prophylactic drugs against bacterial disease [11-14]. ZnO NPs, as a new agent for zinc delivery, in addition to their biotechnological applications, have many implications for the treatment of numerous diseases, including diabetes [15].
The first vitamin discovered in the group of B vitamins was thiamine (B1). In the human body, this vitamin is converted to free thiamine and phosphorylated into different forms, including mono-, di-, tri-, and Pyrophosphate forms, which is the active form of thiamine [16]. One of the most important co-factors in many crucial metabolic processes, including the production of acetylsalicylic acid (COA), the Calvin cycle, and the Pentose phosphate pathway is thiamine pyrophosphate [17]. In the body, high concentrations of thiamine are found in skeletal muscle, heart, liver, kidney, and brain (Martin 2001). The most important functions of thiamine are carbohydrate catabolism and nucleic acid and NADPH production [18]. most important functions of thiamine are carbohydrate catabolism and nucleic acid and NADPH production [19]. It has been also shown that thiamine can stabilize the membrane ion channels and change their activity [20]. The application of thiamine in the treatment of neurological disorders and seizures has been revealed and proven for the healing of diabetic complications. Studies on laboratory animals showed that there is a logical relationship between thiamine and pre-synaptic acetylcholine release [21].
The expansion of nanotechnology in various fields, including medicine, creates the need to investigate the side effects of these materials in the living environment. The present study aimed to evaluate the preventive effects of two different concentrations from ZnO NPs (0.1 and 0.5) alone and along with thiamine against diabetes-induced skin changes in a mouse model.
Animals
In this experimental study, 54 healthy male BALB/C mice, weighing about 22 ± 1g and 5 weeks old (each group 8 samples) were selected. As an adaptation period, animals were kept for a week at a temperature of 19 °C ± 2 °C with a 12-hour dark-light cycle. In order to provide a convenient environment during the experiment period, cage floors were covered with fresh and soft sawdust, and every three days the cages were cleansed to maintain hygiene. The animals were prepared with commercial mouse pellet food containing essential vitamins required for the body besides free access to water. The commercial mouse pellet diet was purchased from Experimental Animal Center in Tehran, Iran. All ethical regulations and protocols on laboratory animal research were considered and approved by the Animal Rights Committee of the Shahrekord Veterinary Faculty.
Preparation of ZnO Nanoparticles
ZnO nanoparticles gained from US Research Nanomaterials, Inc., (Houston, TX, 77084, USA). This nanoparticle (ZnO) harvest itself was a white powder with an amount of purity ≥ 99 + %. The actual characteristics of ZnO nanoparticles refer to their basic physical properties. Nanoparticles of ZnO had dimensions of 10 nm - 30 nm in size (average 20 nm), as listed in Table 1, and were used in concentrations of 0.1 and 0.5 mg/kg (Table 1) [3].
| Table 1: The principal physical characteristics of the ZnO NPs (production reported). | |
| ASP | 10 nm - 30 nm |
| Crystal phase | Single crystal |
| Color | White |
| Morphology | Nearly spherical |
| True density | 5.606g/cm3 |
| SSA | 20-60 m2/g |
Grouping and sampling
After a week of adaptation to the laboratory environment, the mice were randomly divided into 9 groups of 6 individuals each. The diabetes was induced in the experimental mice (groups 5 to 9) by using a single intraperitoneal injection (IP) at a dose of 180 mg/kg body weight of alloxan monohydrate (Sigma-Aldrich, St. Louis, MO, USA) in PBS (pH = 7) soluble [3]. Alloxan is widely used in inducing diabetes in vitro in animal models. This substance causes the destruction of pancreatic beta cells with complex mechanisms. We used alloxan (180 mg/kg body) as in our previous study alloxan was proven to induce diabetes [3].
Seventy-two hours later, a few mice were randomly selected from diabetic mice, and their fasting blood glucose was measured. Mice with a blood glucose level of above 200 mg/dl [3] were noticed as diabetic mice. In order to confirm experimentally induced diabetes, biopsy samples were prepared from the pancreas of mice and were evaluated by a pathologist and confirmed.
After diabetes confirmation, ZnO nanoparticles were administrated to the diabetic mice at two doses of 0.1 and 0.5 mg/kg, and thiamine was injected at a dose of 5 mg/100 g. Finally, all mice were divided into the following groups: All the injections were intraperitoneal (IP).
Group I: Control group; distilled water administered.
Group II: Thiamine (5 mg/100 g)
Group III: ZnO nanoparticles 0.1 mg/kg
Group IV: ZnO nanoparticles 0.5 mg/kg
Group V: Diabetic group (injection of alloxan in doses 220 mg/kg)
Group VI: Diabetes + ZnO nanoparticles 0.1 mg/kg
Group VII: Diabetes + ZnO nanoparticles 0.5 mg/kg
Group VIII: Diabetes + ZnO nanoparticles 0.1 mg/kg along with thiamine (5 mg/100 g)
Group IX: Diabetes + ZnO nanoparticles 0.5 mg/kg along with thiamine (5 mg/100 g)
On the 20th day after the last injection, the mice were anesthetized (with fasting) by chloroform (Merck KGaA, Index No: 2806-18-9), and the blood samples were withdrawn directly from the ventricle of the heart. Blood was centrifuged (Hetich-Germany) for 10 min at 3000 r/min and 4 °C, and the supernatant solution was reserved at -70 °C until biochemical analysis.
The skin samples at the three areas mid-dorsal, mid-ventral, and lateral were considered. After shaving the long hair, the skin from the mentioned regions was excised and trimmed into small-size pieces, fixed in a 10% buffered formalin (Merck) solution for histo-morphometric examinations. Tissue sections of about 5μm were serially obtained, and stained with H&E evaluated under the light microscope.
Stereological evaluation
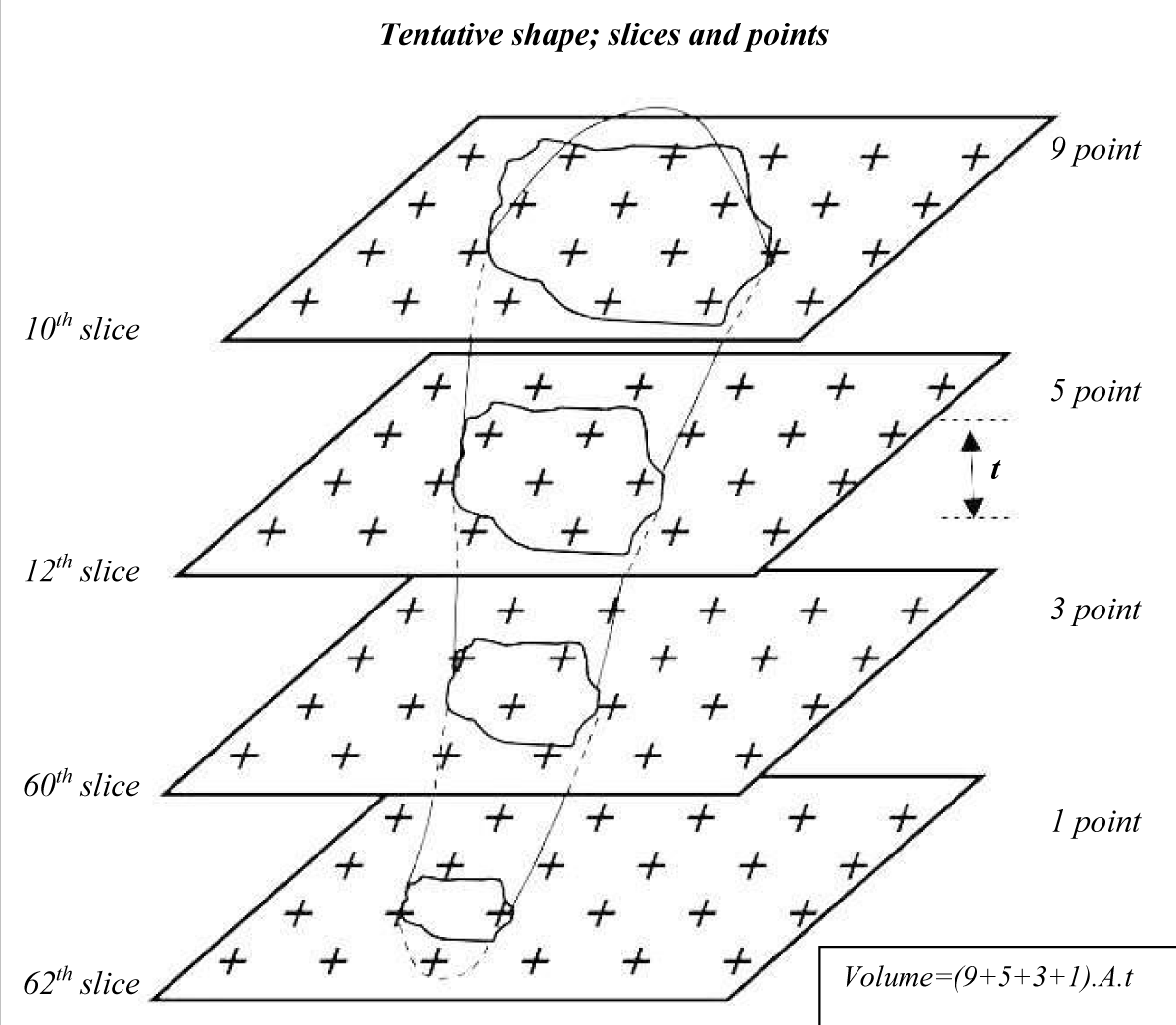
According to the table of random numbers, in the interval between the 50 sections the first section, or primary section (e.g., the 10th section), and the second section that is reference section were selected. Sampling in the distance between sections was so that each 50th section was sampled (the primary sections). Therefore, the primary and reference sections, e.g. 10, 12, 60, 62 were sampled in the entire skin; as detailed in our previous research (Figure 1) [22].
Figure 1: Illustration of the sampling scheme of skin tissue sections. Systematic random samples from skin slices superimposed by a grid of the point square to obtain the volume of uniform random samples. Samples were prepared by a series of parallel cutting plates (serially sections) with slice thickness t distance. The number of serial sections on which the point grid is superimposed includes four slices.
To evaluate the volume densities of tissue collagen bundles and fibroblast, a network of uniformly and permanently spaced points (point grid) was superimposed at random over the image of each predicted section. Collagen bundles that were hit by a point were sampled and subjected to volume densities estimation according to the number of points that hit with the bundles. The total volume of collagen and fibroblasts were calculated using the following formula [23]:
Where Asec is the area of cut surfaces (1st -nth slice) of systematic-random sections through the structure, and t, is the thickness of the sections.
Measurement of serum glucose
Serum glucose levels were measured using a glucose analysis kit (Pars Azmoon Co, Tehran, Iran). The principle was based on the bi-enzymatic assay comprised of enzymatic oxidation of glucose to gluconic acid, yielding hydrogen peroxide, and then the reaction of hydrogen peroxide with 4-amino antipyrine and phenol to yield the colorimetric product which is measured at 500 nm. The results were reported as mg/dl.
Estimation of MDA Level
Malondialdehyde (MDA) as the end product of lipid peroxidation reacts with Thiobarbituric acid (TBA) and yields a colored complex that can be measured spectrophotometrically [24]. In this study, 2 ml of TBA reagent containing 0.375% TBA, 15% TCA, and 0.25 mol/L HCl was added to 1 ml of serum from all groups. The mixture was placed in boiling water for 50 min, cooled to room temperature, and centrifuged at 1000 rpm for 10 min. Thereafter, the absorbance of the supernatant was read at the wavelength of 535 nm against the blank reference. The results were calculated by using a molar extinction coefficient for MDA of 1.56×105/M/cm. The concentration of MDA was expressed as nmol/ml [25].
Nitric oxide assay
Briefly, 1 ml of serum was deproteinized by adding ten microliters of 1.5 g/mL ZnSO4 solution. The mixture was vortexed and centrifuged at 10000 × g for 10 min at 4 °C. Then, 100 ul of the supernatant was added to a 96-well ELISA plate, and each well was submitted to 100 μL of vanadium (III) chloride (8mg/ml) which converts nitrate to nitrite. After the addition of 100 ul of Griess reagent (an equal mixture of 1% sulphanilamide in 5% phosphoric acid and 0.1% N-(1-naphthyl) ethylenediamine hydrochloride in distilled water), the plates were maintained at room temperatures for 30 minutes and the nitrite production was quantified calorimetrically at 540 nm. The NO levels were calculated using various concentrations of sodium nitrite (0.1-100 μm) as a standard and expressed as μmol/ml [26].
Measurement of serum BUN and Cr levels
Serum BUN was measured using a commercial kit (Pars Azmoon Co., Tehran, Iran) based on the enzymatic hydrolysis of urea to ammonia by urease. Then, in a parallel reaction, the ammonia is converted to glutamate by glutamate dehydrogenase and this reaction was monitored at 340 nm. The Cr level was measured by the Jaffe method (Pars. Azmoon Kit, Tehran, Iran) in which creatinine reacts with picric acid to form a reddish complex [27].
GGT assay
Gamma-glutamyl transferase (GGT) activity was measured by using the Pars Azmoon kit (Tehran, Iran, according to the manufacturer). The reaction is evaluated based on transferring of the γ-glutamyl group from γ-glutamyl-p-nitroanilide to acceptor glycylglycine and the production of para-nitroaniline which can be determined calorimetrically at 405 nm; the results were reported as U/l.
Statistical analysis
Statistical analyses of volume, histo-morphometric and biochemical data were carried out using the SPSS statistical software package version 23.0 (SPSS Inc., Chicago, IL) for Windows. Data are declared as mean ± standard deviation (SD) and statistical variations were tested by One-way ANOVA. LSD Post Hoc test was applied and the values confirming p < 0.05 were considered significant compared to the rest.
Body weight
There were no deaths during and after the investigation due to drug injection.
To evaluate the effect of zinc oxide and thiamine nanoparticles on the weight criterion, the body weight of mice was determined at the beginning of treatment (day 0);
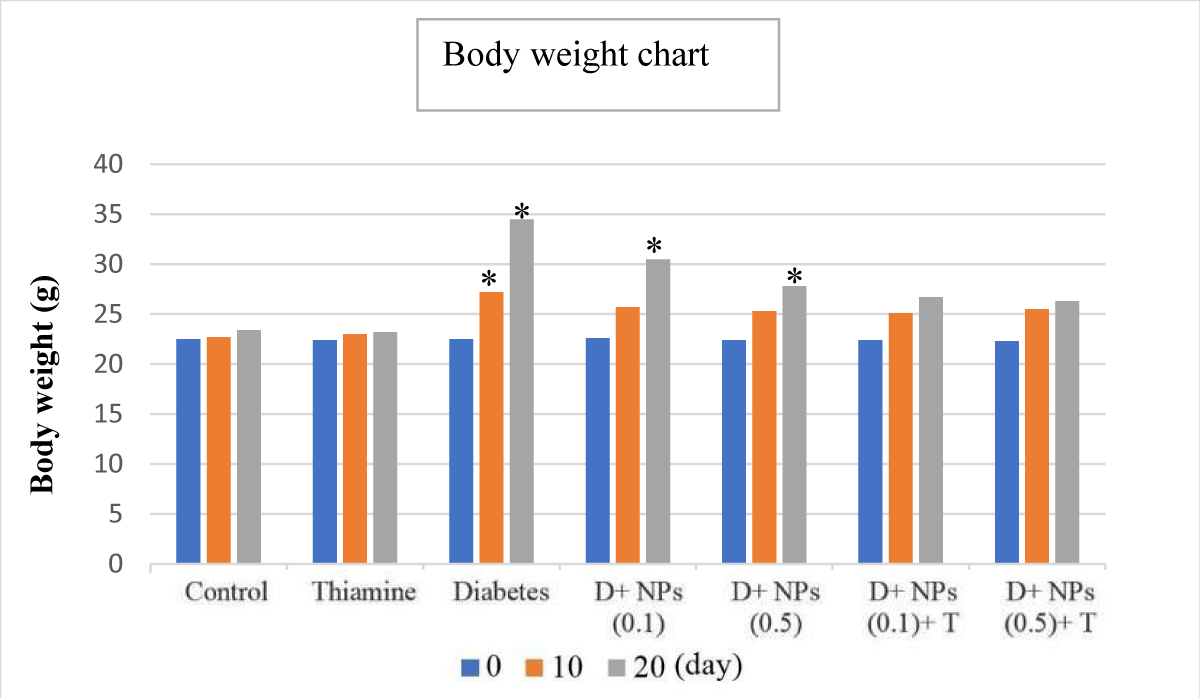
It should be noted that on day 0 there was no significant difference in body weight at the beginning of the experiment from all groups (p > 0.05). There was a significant difference in the untreated diabetic group (group V) on the 10th and 20th days in body weight compared to the control and thiamine (groups I and II, respectively) groups (Figure 2) (p < 0.05). In VI and VII groups (Diabetes+ZnO NPs, 0.1 and 0.5), on the 20th day, the body weight was increased in comparison with controls (control and thiamine groups) (p < 0.05). In VIII and IX groups (Diabetes+ZnO NPs, '0.1 and 0.5'+thiamine), the statistical assessment showed that there were no significant differences on all study days in comparison with the control group (p > 0.05). During the research period, we observed a gradual reduction in body weight in the treated-diabetic mice in contrast to a gain in body weight in the alloxan-induced mice (Figure 2).
Figure 2: The mean changes from day 0 in body weight (g), for all controls plus diabetes (untreated and treated); D: Diabetes; T: Thiamine, NPs: ZnO nanoparticles; mean ± SD (n = 7); *significant difference in each group with day 0 and control, p < 0.05.
Morphometric criteria
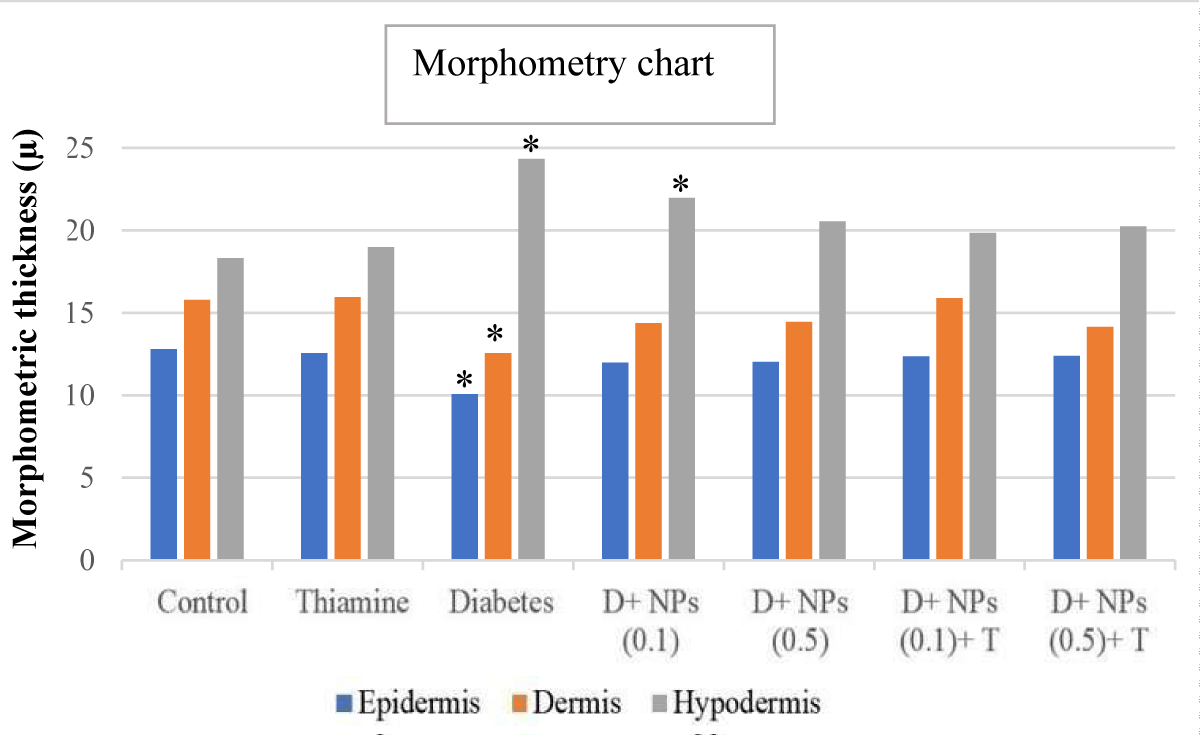
Figure 3 presents findings from the morphometrical analysis of the dermis, epidermis, and hypodermis of skin in the control, untreated, and treated diabetic groups. The morphometric data obtained showed different variations among untreated and treated diabetic groups (groups 5 to 9) than to control groups (groups 1 and 2), according to intended factors. The thickness of the epidermis layer lining the surface of the skin exhibited a significant decrease in the untreated diabetic group (group 5) compared to the control group (p < 0.05). In addition, the diabetic group treated with ZnO NPs (0.1 and 0.5) plus thiamine showed a significant increase in epidermis thickness compared to untreated diabetic mice (p > 0.05), near normal conditions.
Figure 3: The morphometrical parameters of measured in different layers of cutaneous tissue in experimental groups; mean ± SD (n = 7); * significant difference in each parameter with the control group; p < 0.05.
The overall dermis thickness of the skin showed a similar histomorphometry pattern with the epidermis, so the thickness of the dermis layer in the diabetic control group was statistically decreased compared to the control mice (p < 0.05). Furthermore, no significant change was detected in the thickness of the dermis layer in mice receiving ZnO NPs (0.1 and 0.5) alone and along with thiamine (groups 6 to 9) than that of control (p > 0.05).
The mean thickness (μm) of the hypodermis layer in the diabetic and diabetic + ZnO NPs (0.1 mg/kg) groups increased significantly compared to the control group (p < 0.05). Treatment with ZnO NPs and thiamine showed a marked reduction in thickening hypodermis in groups 7 to 9 compared to the diabetic group (p > 0.05).
Histological criteria
Photomicrograph of skin sections in different groups after the 20th day stained with hematoxylin and eosin. The skin tissue of intact and treated mice in different layers of epidermis, dermis, and hypodermis is shown in Figures 4,5.
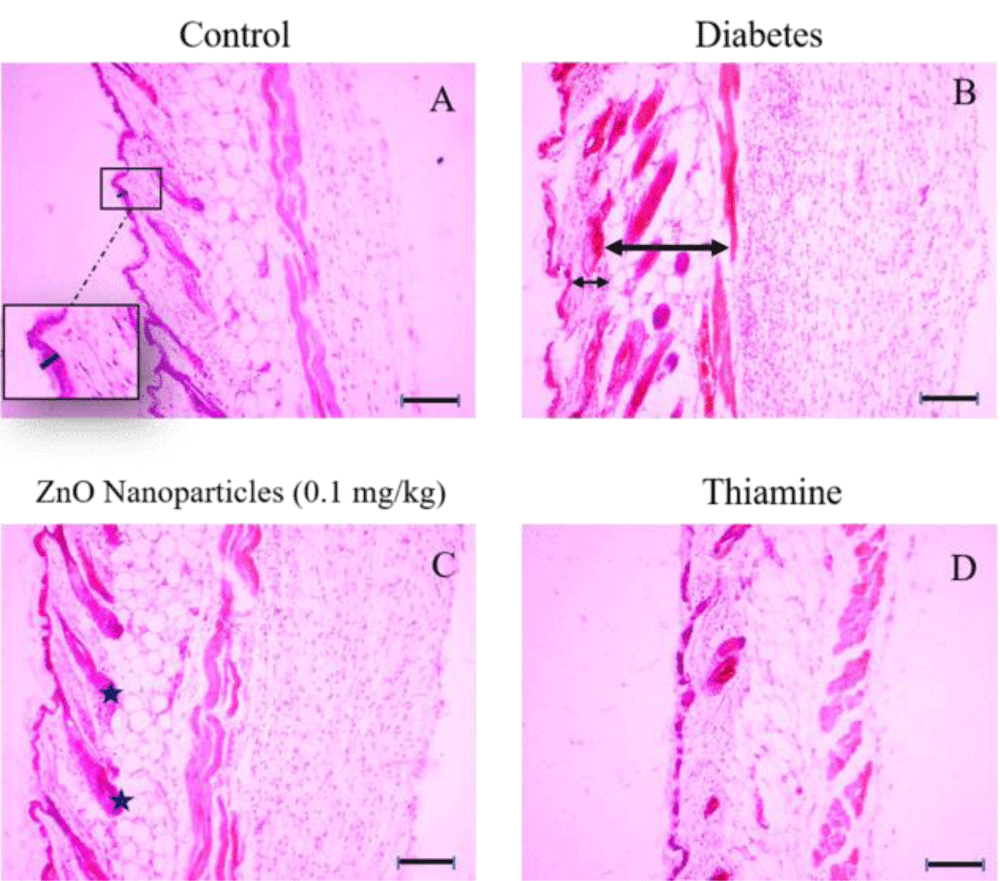
Figure 4: Histologic sections of lumbar area skin of control, diabetes, ZnO nanoparticles (0.1 mg/kg) and thiamine groups stained with Hematoxylin and Eosin (H&E), Bars: 10 µm. A: The thickness of the epidermal part belonging to the control group is shown with a dark blue bar and no significant difference between the control group and other groups is apparent in epidermal thickness. B: The decrease in thickness of the dermal layer in the diabetes group isn’t noticeable (short arrow), whereas the hypodermis layer has increased significantly (long arrow) in comparison with the control group. C: Some of the hair follicles are marked by the asterisks between the dermis and hypodermis layers. D: Photomicrograph belonging to the tissue structure in the thiamine group.
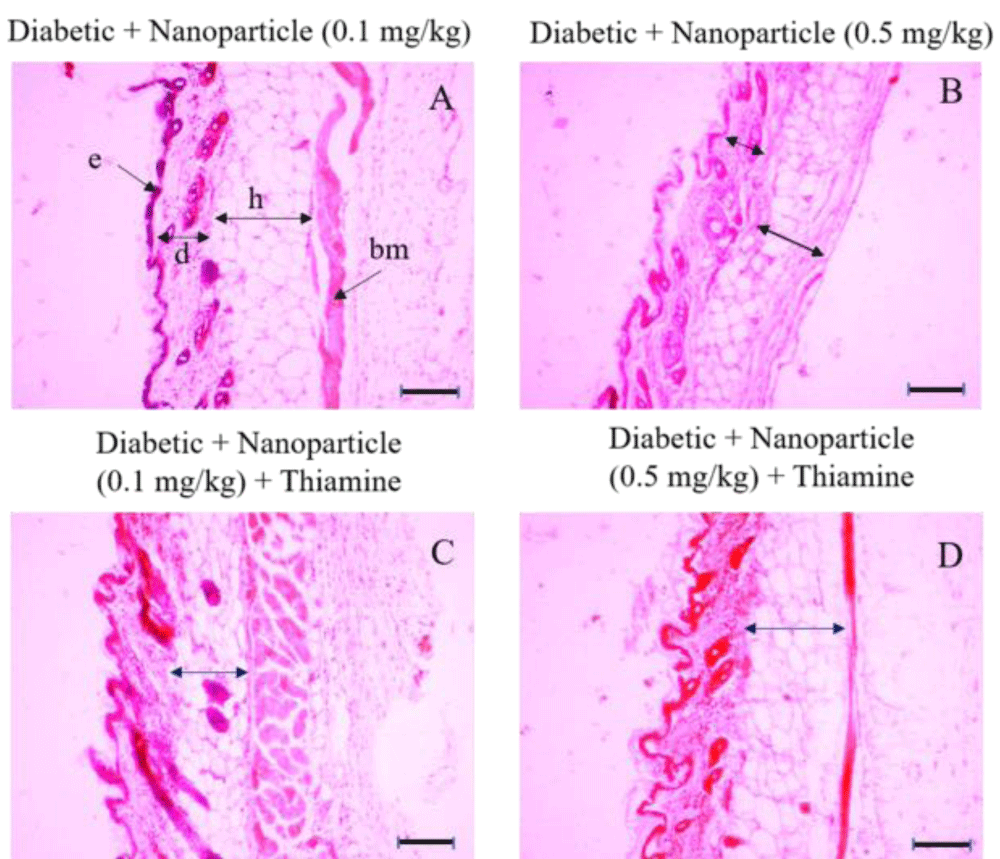
Figure 5: Histologic sections of lumbar area skin of Diabetic+ Nanoparticle (0.1 mg/kg), Diabetic+ Nanoparticle (0.5 mg/kg), Diabetic+ Nanoparticle (0.1 mg/kg) + Thiamine and Diabetic+ Nanoparticle (0.5 mg/kg) + Thiamine groups stained with hematoxylin and eosin (H&E). e: epidermis; d: dermis; h: hypodermis. Bars: 10 µm. There is no significant difference in epidermis between these groups or in comparison to the control group. There was a considerable decrease in the dermal layer of the Diabetic + Nanoparticle (0.5 mg/kg) group (picture B, short arrow) compared to the control group and also the Diabetic + Nanoparticle (0.1 mg/kg) group (picture A, black arrow marked with a [d]). The least thickness of the hypodermis layer was observed in the Diabetic + Nanoparticle (0.5 mg/kg) group (picture B, long arrow) whereas no significant decrease emerged in hypodermis thickness of the groups with the administration of nanoparticles in combination with thiamine (picture C and D).
Volume of collagen bundles
The volume density of collagen bundles (Table 2) in the skin samples of the untreated diabetic mice was significantly lower than those of the control and thiamine individuals (p < 0.05). However, the twenty-day treatment with ZnO NPs (0.1 and 0.5) and thiamine (groups 6 to 9) caused a significant increase in the volume density of collagen when compared to the untreated diabetic group (p < 0.05).
| *Table 2: Quantitative parameter of volume density of collagen bundles (Vv; %), (Mean ± SD). | |||
| Groups | Collagen volume density, % | p value | |
| g I | Control | 37.5a | - |
| g II | Thiamine | 36.8a | - |
| g III | ZnO NPs (0.1 mg/kg) | 36.5a | p > 0.05 |
| g IV | ZnO NPs (0.5 mg/kg) | 36.7a | p > 0.05 |
| g V | Diabetes | 33.5b | p < 0.05 |
| g VI | D + ZnO NPs (0.1 mg/kg) | 36.4a | p > 0.05 |
| g VII | D + ZnO NPs (0.5 mg/kg) | 36.2a | p > 0.05 |
| g VIII | D + ZnO NPs (0.1 mg/kg) + Thiamine | 35.9a | p > 0.05 |
| g IX | D + ZnO NPs (0.5 mg/kg) + Thiamine | 36.2a | p > 0.05 |
| a-bMeans in the same column with different letters are significantly different (p < 0.05); *The table above shows the values measured 20 days after diabetes induction on mice; D: Diabetes. | |||
Table 3 summarizes the level of blood glucose (mg/dl), Cr (mg/dl), BUN (mg/dl), and GGT (u/l) of controls and experimental animals. When all the mentioned biochemical parameters were evaluated in the control group, it was found that there was no significant difference with the thiamine group (p > 0.05).
| *Table 3: The amounts of blood factors (means ± SD) in all the groups during the study period. | |||||
| Groups | Glucose (mg/dl) | Cr | BUN | GGT | |
| (mg/dl) | (mg/dl) | (U/L) | |||
| g I | Control | 82.33 ± 4.10a | 0.23 ± 0.02a | 43.83 ± 5.89a | 6.31 ± 0.65a |
| g II | Thiamine | 80.80 ± 3.61a | 0.21 ± 0.03a | 44.40 ± 5.47a | 6.42 ± 0.57a |
| g III | ZnO NPs (0.1 mg/kg) | 81.25 ± 3.4a | 0.24 ± 0.03a | 41.25 ± 4.54a | 5.90 ± 0.48a |
| g IV | ZnO NPs (0.5 mg/kg) | 80.90 ± 3.8a | 0.23 ± 0.03a | 43.80 ± 4.25a | 6.40 ± 0.55a |
| g V | Diabetes | 351.32 ± 4.17b | 0.89 ± 0.12b | 86.74 ± 7.10b | 12.15 ± 1.56b |
| g VI | D + ZnO NPs (0.1 mg/kg) | 150.87 ± 5.51c | 0.52 ± 0.02c | 63.62 ± 5.82c | 9.91 ± 1.09c |
| g VII | D + ZnO NPs (0.5 mg/kg) | 162.66 ± 3.67c | 0.47 ± 0.03c | 67.50 ± 6.93c | 8.89 ± 0.91c |
| g VIII | D + ZnO NPs (0.1 mg/kg) + Thiamine | 123.16 ± 7.38c | 0.36 ± 0.02c | 55.23 ± 5.36c | 8.06 ± 0.87c |
| g IX | D + ZnO NPs (0.5 mg/kg) + Thiamine | 114.2 ± 4.63c | 0.32 ± 0.02c | 54.42 ± 5.34c | 7.57 ± 0.86c |
| a–c Means in the same column with different letters are significantly different (p < 0.05); *The above table shows values measured 20 days after induction of diabetes in mice; D: Diabetes. | |||||
Fasting blood glucose
The blood glucose level of the untreated diabetic group (5th group) was significantly (p < 0.05) higher than the control group.
Injection of the ZnO NPs (0.1 and 0.5) alone and plus thiamine into the diabetic rats increased the blood glucose concentration sharply (p < 0.05).
Cr, BUN, GGT values
A similar pattern was observed among Cr, BUN, and GGT levels when all groups in each parameter were compared. Plasma Cr, BUN, and GGT values were elevated significantly in untreated diabetic mice compared with controls (p < 0.05). However, the doses of 0.1 and 0.5 mg/kg ZnO NPs alone and along with thiamine (groups 6 to 9) produced a significant decrease (p < 0.05) in the serum levels of Cr, BUN, and GGT than to control and untreated diabetic groups. However, the biochemical experiments were not significantly changed between treated diabetic groups 6 to 9, (p > 0.05) (Table 3).
MDA and NO levels
Table 4 displayed that in the untreated diabetic mice (group 5), the MDA value was significantly higher than the control mice (5.97 ± 0.62 and 3.29 ± 0.38 nmol/ml, respectively) (p < 0.05). Treatment with ZnO NPs (0.1 and 0.5 mg/kg) alone and along with thiamine decreased the MDA value of the serum compared with the untreated diabetic mice, however, these changes were significant. (p < 0.05). A significant increase in the skin amount of NO was seen in the untreated diabetic mice compared with the control group (p < 0.05). In diabetic mice treated with ZnO NPs (0.1 and 0.5) alone and in combination with thiamine (groups 6 to 9), serum NO concentration was lower than (0.80 ± 0.05 nmol/ml) untreated diabetic mice and higher than in the control group (p > 0.05).
*Table 4: The concentrations of MDA and NO in all the groups (means ± SD). |
||||
| Groups | MDA (nmol/ml) | NO (µmol/ml) | p value | |
| g I | Control | 3.29 ± 0.38a | 0.21 ± 0.04a | - |
| g II | Thiamine | 3.31 ± 0.47a | 0.22 ± 0.03a | - |
| g III | ZnO NPs (0.1 mg/kg) | 3.72 ± 0.41a | 0.22 ± 0.04a | p > 0.05 |
| g IV | ZnO NPs (0.5 mg/kg) | 3.31 ± 0.30a | 0.27 ± 0.04 a | p > 0.05 |
| g V | Diabetes | 5.97 ± 0.62b | 0.80 ± 0.05b | p < 0.05 |
| g VI | D + ZnO NPs (0.1 mg/kg) | 4.86 ± 0.44a | 0.45 ± 0.04a | p > 0.05 |
| g VII | D + ZnO NPs (0.5 mg/kg) | 4.72 ± 0.35a | 0.42 ± 0.03a | p > 0.05 |
| g VIII | D + ZnO NPs (0.1 mg/kg) + Thiamine | 4.01 ± 0.33a | 0.40 ± 0.05a | p > 0.05 |
| g IX | D + ZnO NPs (0.5 mg/kg) + Thiamine | 3.85 ± 0.32a | 0.38 ± 0.04a | p > 0.05 |
a–b Means in the same column with different letters are significantly different (p < 0.05); *The table above shows the values measured 20 days after diabetes induction on mice; D: Diabetes. |
||||
Cutaneous complications of diabetes have attracted a lot of attention because of the clinically remarkable problems, to the extent that it leads to a reduction in skin thickness and increased blood glucose levels [28]. Since then, the balance between the harms and benefits of metal-based nanoparticles has been discussed, and their effectiveness in dealing with the harmful effects of some diseases, including diabetes, has been more evident. These investigations tended to objectify that nanoparticles, such as zinc oxide, could exhibit anti-glycemic activity with administered treatment for different planned periods [29,30]. Our research revealed that alloxan-induced diabetes in mice significantly changes the histological structure of the skin of treated animals compared to non-diabetic animals. Diabetic mice, when compared with non-diabetic mice, showed clinical signs of diabetes such as high fasting blood sugar, polyuria, and polydipsia (by comparing their body weight) (351.32 ± 4.17 versus 82.33 ± 4.10) (p < 0.05).
A specific hallmark that facilitates molecular diagnosis in the evaluation of diabetes is glycation formation; glycation is a non-enzymatic chemical reaction of free reducing sugars, in which reducing sugar linkage with a free amino group of proteins [31]. These irreversible reactions produce advanced glycation end products (AGEs) with the help of a chain of various reactions [32]. Some AGEs that may use as a glycation stress indicator, and a marker for diabetes, included CML (Nε -(carboxymethyl) lysine) [33]. As a consequence, the product of protein modification by glyoxal (CML) is observed when conditions lead to high oxidative stress and/or in diabetic situations. These results proved the fact that CML can transform skin collagen by reducing skin elasticity and elasticity and leading to skin shrinkage [34].
The results of the present study showed that diabetes decreased the collagen volume density (%) in mice's skin compared to healthy mice. Besides, in groups of the ZnO NPs (0.1 and 0.5) alone and along with thiamine, the collagen volume decreased more than those in the control group. Nevertheless, the effect of untreated diabetes was more severe than the effect of ZnO NPs (0.1 and 0.5) alone and along with thiamine on the collagen volume. ZnO NPs and thiamine had a controlling role in further reducing collagen volume in the diabetic group. Concerning the ZnO NPs, our results pointed out that its administration in combination with thiamine has more therapeutic effects than when used alone. Angela, et al. (2016) in their research, showed that the level of matrix metalloproteinases (MMPs) in the skin of diabetics is greatly increased [35]. Mittal, et al. (2016) demonstrated MMPs are a group of proteinases, because of the nature of their substrate, contain six subclasses, including collagenase [36]. Studies conducted in various aspects by researchers over several years have proven that MMP-1 (collagenase) is significantly increased in diabetic skin or normal aging skin [37]. These investigations led to the conclusion that MMP-1 is the major protease capable of initiating the fragmentation of native fibrillar collagen [38].
Collagen assists the molecular constructions in developed and organized tissues, such as the skin, to maintain their morphological and mechanical properties. Since the consistency, integrity, and longevity of the dermis depend on collagen, therefore, the quality characteristics of collagen can affect these indicators [39]. The present study is consistent with the previous studies because the thickness per micrometer of different layers of the epidermis and dermis in the skin of untreated diabetic animals showed noticeable changes and when compared with the control group, it showed a significant decrease. gave In the group of zinc oxide nanoparticles (0.1 and 0.5) alone and in combination with thiamine, the decrease in thickness was partially compensated and showed a significant increase compared to the untreated diabetic group.
The results of this research showed that thiamine decreased blood glucose and Cr, BUN, and GGT values as well as MDA and NO in the treatment groups compared to the diabetes group. The available evidence indicated that oxidative stress increases in diabetic patients due to excessive production of reactive oxygen species (Ros), which in turn reduces the efficiency of antioxidant defense [40]. Oxidation of other macromolecules and proteins occurs during the development of diabetes. Mutations in mitochondrial DNA have also been reported in diabetic tissues, which is a type of oxidative stress related to mitochondrial damage [41].
Thiamine regulates basal metabolism in the form of coenzymes and (in some cases) non-coenzymes. One of the most prominent coenzymes of thiamine is the Pyruvate dehydrogenase complex (PDC) which is in the bioenergetic processes. The essential role of PDC in cellular metabolism and most of its clinical features is revealed following its deficiency, for example, mental retardation, ataxia, peripheral neuropathy, and structural abnormalities of the brain [42,43]. Studies show that pathological accumulation of reactive oxygen species in cells is associated with PDC deficiency. Mitochondrial manganese superoxide dismutase activity is severely reduced in PDC-deficient cells. In spite of the precise action mechanism of thiamine against lead toxicity is unclear, it may be related to the formation of complexes between thiamine and lead, followed by its excretion. Thiamine has been suggested to facilitate the removal of lead from body fluids and other tissues by the formation of excretory complexes [44].
Zinc has been shown to partially improve glucose metabolism through insulin signal transduction by increasing hepatic glycogenesis. In administering zinc complexes orally in the body, a tendency to reduce blood glucose is also observed [45]. In the present study, intraperitoneally administration of ZnO NPs alone and along with thiamine displayed that values of serum parameters of fasting blood glucose, Cr, BUN, GGT, MDA, and NO improved compared to the untreated diabetes group. However, hyperglycemia manifested its effects by increasing Cr, BUN, GGT, MDA, and NO levels, as a destructive agent. Some studies have been presented about the acute effects of high-dose ZnONPs on circulating blood glucose levels and have been shown that high doses of ZnONPs (e.g. 10 mg/kg) can limit the therapeutic utilizations at diabetic patients [30]; however, the antidiabetic effects of ZnONPs in low doses have also been confirmed [3]. As the present study showed, the use of ZnO NPs in low doses (0.1 and 0.5) was useful as an anti-hyperglycemic agent and effective in controlling the destructive effects of diabetes.
The available data about the mechanism of diabetes effect on thiamine processes are not complete and the precise mechanism is unknown; however, some research has suggested some mechanisms. Among the several enzymes involved in carbohydrate metabolism, thiamine (vitamin B1) is an essential factor that plays a vital role in carbohydrate metabolism [46]. Rosner, et al. (2015) showed that diabetes causes insulin deficiency, and following, the rate of thiamine transfer among the intestine is reduced. They found that rats with insulin deficiency showed noticeably reduced transfer of free thiamine and monophosphate [47]. On the contrary, when there is a decrease in the amount of thiamine (both intake and absorption), insulin synthesis and secretion are significantly disturbed [48].
Hyperglycemia as a definitive marker in the challenge with normal conditions altered serum biochemical parameters and stereological factors in the skin. According to the stereological and biochemical findings, from one-dimension ZnO NPs and another dimension thiamine, were shown to act as anti-diabetic agents in combination together. It is suggested to use other vitamins in the investigation of the therapeutic effects of diabetes.
Declarations
Ethical approval: The project was conducted in accordance with animal welfare standards for the species. Experimental procedures were approved by the animal care and ethics committee of Shahrekord University (IR.SKU.REC.1400.015).
Competing interests: All authors read and approved the final submitted article. None of the authors have conflicts of interest.
Authors' contributions: RF carefully monitored all aspects of this study from the idea to the presentation of the article; SP conducted this research. RF and BK analyzed the data and also wrote the paper; BK and SH contributed to the immunochemical analyses.
Funding: The project was supported by the Faculty of Veterinary Medicine, Vice Chancellor for Research, Shahrekord University.
Availability of data and materials: Data are all contained within the paper and are also available from the corresponding author upon reasonable request.
- Mahan LK, Escott-Stump S. Krause's food, nutrition, & diet therapy, Saunders Philadelphia, 2004.
- Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997 Jul;20(7):1183-97. doi: 10.2337/diacare.20.7.1183. PMID: 9203460.
- Amiri A, Dehkordi RAF, Heidarnejad MS, Dehkordi MJ. Effect of the Zinc Oxide Nanoparticles and Thiamine for the Management of Diabetes in Alloxan-Induced Mice: a Stereological and Biochemical Study. Biol Trace Elem Res. 2018 Feb;181(2):258-264. doi: 10.1007/s12011-017-1035-x. Epub 2017 May 22. PMID: 28534098.
- Ahmadi A, Hasanzadeh J, Rahimi Madiseh M, Lashkari L. Effective factors in the quality of life in patients with type 2 diabetes in Chaharmahal & Bakhteyari Province. Journal of north Khorasan University of medical sciences. 2011; 3:7-13.
- Taylor KR, Costanzo AE, Jameson JM. Dysfunctional γδ T cells contribute to impaired keratinocyte homeostasis in mouse models of obesity. J Invest Dermatol. 2011 Dec;131(12):2409-18. doi: 10.1038/jid.2011.241. Epub 2011 Aug 11. PMID: 21833015; PMCID: PMC3213272.
- Chen X, Lin W, Lu S, Shi Y, Zou J, Liu Z, Liao W. Insulin prevents latent skin lesions by inhibiting the generation of advanced glycation end products in streptozotocin-induced diabetic rats. Endocr Pathol. 2009 Fall;20(3):163-9. doi: 10.1007/s12022-009-9084-0. PMID: 19488861.
- Cavallini M. Autologous fibroblasts to treat deep and complicated leg ulcers in diabetic patients. Wound Repair Regen. 2007 Jan-Feb;15(1):35-8. doi: 10.1111/j.1524-475X.2006.00182.x. PMID: 17244317.
- Schmidt-Mende L, MacManus-Driscoll JL. ZnO–nanostructures, defects, and devices, Materials today. 2007; 10:40-48.
- Odendaal JP, Reinecke AJ. Quantitative assessment of effects of zinc on the histological structure of the hepatopancreas of terrestrial isopods. Arch Environ Contam Toxicol. 2007 Oct;53(3):359-64. doi: 10.1007/s00244-006-0080-9. Epub 2007 Jul 4. PMID: 17612782.
- Juzenas P, Chen W, Sun YP, Coelho MA, Generalov R, Generalova N, Christensen IL. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv Drug Deliv Rev. 2008 Dec 14;60(15):1600-14. doi: 10.1016/j.addr.2008.08.004. Epub 2008 Sep 20. PMID: 18840487; PMCID: PMC2695009.
- Rzigalinski BA, Strobl JS. Cadmium-containing nanoparticles: perspectives on pharmacology and toxicology of quantum dots. Toxicol Appl Pharmacol. 2009 Aug 1;238(3):280-8. doi: 10.1016/j.taap.2009.04.010. Epub 2009 Apr 18. PMID: 19379767; PMCID: PMC2709954.
- Li J, Guo D, Wang X, Wang H, Jiang H, Chen B. The Photodynamic Effect of Different Size ZnO Nanoparticles on Cancer Cell Proliferation In Vitro. Nanoscale Res Lett. 2010 Apr 16;5(6):1063-71. doi: 10.1007/s11671-010-9603-4. PMID: 20671778; PMCID: PMC2893699.
- Heinlaan M, Ivask A, Blinova I, Dubourguier HC, Kahru A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere. 2008 Apr;71(7):1308-16. doi: 10.1016/j.chemosphere.2007.11.047. Epub 2008 Jan 14. PMID: 18194809.
- Chu D, Gao J, Wang Z. Neutrophil-Mediated Delivery of Therapeutic Nanoparticles across Blood Vessel Barrier for Treatment of Inflammation and Infection. ACS Nano. 2015 Dec 22;9(12):11800-11. doi: 10.1021/acsnano.5b05583. Epub 2015 Nov 5. PMID: 26516654; PMCID: PMC4699556.
- Siddiqui SA, Or Rashid MM, Uddin MG, Robel FN, Hossain MS, Haque MA, Jakaria M. Biological efficacy of zinc oxide nanoparticles against diabetes: a preliminary study conducted in mice. Biosci Rep. 2020 Apr 30;40(4):BSR20193972. doi: 10.1042/BSR20193972. PMID: 32207527; PMCID: PMC7138905.
- Fattal I, Friedmann N, Fattal-Valevski A. The crucial role of thiamine in the development of syntax and lexical retrieval: a study of infantile thiamine deficiency. Brain. 2011 Jun;134(Pt 6):1720-39. doi: 10.1093/brain/awr068. Epub 2011 May 9. PMID: 21558277.
- Tunc-Ozdemir M, Miller G, Song L, Kim J, Sodek A, Koussevitzky S, Misra AN, Mittler R, Shintani D. Thiamin confers enhanced tolerance to oxidative stress in Arabidopsis. Plant Physiol. 2009 Sep;151(1):421-32. doi: 10.1104/pp.109.140046. Epub 2009 Jul 29. PMID: 19641031; PMCID: PMC2735988.
- Singleton CK, Martin PR. Molecular mechanisms of thiamine utilization. Curr Mol Med. 2001 May;1(2):197-207. doi: 10.2174/1566524013363870. PMID: 11899071.
- Rapala-Kozik M. Vitamin B1 (Thiamine): a cofactor for enzymes involved in the main metabolic pathways and an environmental stress protectant, Advances in botanical research. Elsevier. 2011; 37-91.
- Bâ A. Metabolic and structural role of thiamine in nervous tissues. Cell Mol Neurobiol. 2008 Nov;28(7):923-31. doi: 10.1007/s10571-008-9297-7. Epub 2008 Jul 19. PMID: 18642074.
- Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer's disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi: 10.1002/14651858.CD001498. PMID: 11405995.
- Fatahian Dehkordi RA, Moradi H. Stereological estimation and morphological assessment of the endocrine pancreatic components in relation to sex in hen. Vet Res Forum. 2015 Winter;6(1):49-54. Epub 2015 Mar 15. PMID: 25992251; PMCID: PMC4405685.
- Inuwa IM. First-order stereology in diabetes and endocrine research-number and volume estimation of objects. International journal of Diabetes and Metabolism. 2005; 13:10.
- Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. Epub 2014 May 8. PMID: 24999379; PMCID: PMC4066722.
- Yazdi HB, Hojati V, Shiravi A, Hosseinian S, Vaezi G, Hadjzadeh MA. Liver Dysfunction and Oxidative Stress in Streptozotocin-Induced Diabetic Rats: Protective Role of Artemisia Turanica. J Pharmacopuncture. 2019 Jun;22(2):109-114. doi: 10.3831/KPI.2019.22.014. Epub 2019 Jun 30. PMID: 31338251; PMCID: PMC6645339.
- Kalugalage T, Rodrigo C, Vithanage T, Somaratne P, De Silva HJ, Handunnetti S, Rajapakse S. Low serum total nitrite and nitrate levels in severe leptospirosis. BMC Infect Dis. 2013 May 6;13:206. doi: 10.1186/1471-2334-13-206. PMID: 23648003; PMCID: PMC3651868.
- Syal K, Srinivasan A, Banerjee D. Streptomycin interference in Jaffe reaction - possible false positive creatinine estimation in excessive dose exposure. Clin Biochem. 2013 Jan;46(1-2):177-9. doi: 10.1016/j.clinbiochem.2012.10.031. Epub 2012 Oct 31. PMID: 23123914.
- Ahmed F, Husain Q, Ansari MO, Shadab G. Antidiabetic and oxidative stress assessment of bio-enzymatically synthesized zinc oxide nanoformulation on streptozotocin-induced hyperglycemic mice. Applied Nanoscience. 2020; 10:879-893.
- Bayrami A, Parvinroo S, Habibi-Yangjeh A, Rahim Pouran S. Bio-extract-mediated ZnO nanoparticles: microwave-assisted synthesis, characterization and antidiabetic activity evaluation. Artif Cells Nanomed Biotechnol. 2018 Jun;46(4):730-739. doi: 10.1080/21691401.2017.1337025. Epub 2017 Jun 15. PMID: 28617629.
- Virgen-Ortiz A, Apolinar-Iribe A, Díaz-Reval I, Parra-Delgado H, Limón-Miranda S, Sánchez-Pastor EA, Castro-Sánchez L, Jesús Castillo S, Dagnino-Acosta A, Bonales-Alatorre E, Rodríguez-Hernández A. Zinc Oxide Nanoparticles Induce an Adverse Effect on Blood Glucose Levels Depending On the Dose and Route of Administration in Healthy and Diabetic Rats. Nanomaterials (Basel). 2020 Oct 12;10(10):2005. doi: 10.3390/nano10102005. PMID: 33053624; PMCID: PMC7599450.
- Kim CS, Park S, Kim J. The role of glycation in the pathogenesis of aging and its prevention through herbal products and physical exercise. J Exerc Nutrition Biochem. 2017 Sep 30;21(3):55-61. doi: 10.20463/jenb.2017.0027. PMID: 29036767; PMCID: PMC5643203.
- Hafizur RM, Momin S, Fatima N. Prevention of advanced glycation end-products formation in diabetic rats through beta-cell modulation by Aegle marmelos. BMC Complement Altern Med. 2017 Apr 21;17(1):227. doi: 10.1186/s12906-017-1743-y. PMID: 28431540; PMCID: PMC5399853.
- Li M, Zeng M, He Z, Zheng Z, Qin F, Tao G, Zhang S, Chen J. Effects of Long-Term Exposure to Free Nε-(Carboxymethyl)lysine on Rats Fed a High-Fat Diet. J Agric Food Chem. 2015 Dec 30;63(51):10995-1001. doi: 10.1021/acs.jafc.5b05750. Epub 2015 Dec 18. PMID: 26652688.
- Hansen F, Battú CE, Dutra MF, Galland F, Lirio F, Broetto N, Nardin P, Gonçalves CA. Methylglyoxal and carboxyethyllysine reduce glutamate uptake and S100B secretion in the hippocampus independently of RAGE activation. Amino Acids. 2016 Feb;48(2):375-85. doi: 10.1007/s00726-015-2091-1. Epub 2015 Sep 7. PMID: 26347375.
- Argyropoulos AJ, Robichaud P, Balimunkwe RM, Fisher GJ, Hammerberg C, Yan Y, Quan T. Alterations of Dermal Connective Tissue Collagen in Diabetes: Molecular Basis of Aged-Appearing Skin. PLoS One. 2016 Apr 22;11(4):e0153806. doi: 10.1371/journal.pone.0153806. PMID: 27104752; PMCID: PMC4841569.
- Mittal R, Patel AP, Debs LH, Nguyen D, Patel K, Grati M, Mittal J, Yan D, Chapagain P, Liu XZ. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. J Cell Physiol. 2016 Dec;231(12):2599-621. doi: 10.1002/jcp.25430. Epub 2016 Jul 11. PMID: 27187048.
- Cathcart J, Pulkoski-Gross A, Cao J. Targeting Matrix Metalloproteinases in Cancer: Bringing New Life to Old Ideas. Genes Dis. 2015 Mar 1;2(`1):26-34. doi: 10.1016/j.gendis.2014.12.002. PMID: 26097889; PMCID: PMC4474140.
- Hong YH, Kim D, Nam G, Yoo S, Han SY, Jeong SG, Kim E, Jeong D, Yoon K, Kim S, Park J, Cho JY. Photoaging protective effects of BIOGF1K, a compound-K-rich fraction prepared from Panax ginseng. J Ginseng Res. 2018 Jan;42(1):81-89. doi: 10.1016/j.jgr.2017.01.002. Epub 2017 Jan 10. PMID: 29348726; PMCID: PMC5766695.
- Volksdorf T, Heilmann J, Eming SA, Schawjinski K, Zorn-Kruppa M, Ueck C, Vidal-Y-Sy S, Windhorst S, Jücker M, Moll I, Brandner JM. Tight Junction Proteins Claudin-1 and Occludin Are Important for Cutaneous Wound Healing. Am J Pathol. 2017 Jun;187(6):1301-1312. doi: 10.1016/j.ajpath.2017.02.006. Epub 2017 Apr 12. PMID: 28412298.
- Kayama Y, Raaz U, Jagger A, Adam M, Schellinger IN, Sakamoto M, Suzuki H, Toyama K, Spin JM, Tsao PS. Diabetic Cardiovascular Disease Induced by Oxidative Stress. Int J Mol Sci. 2015 Oct 23;16(10):25234-63. doi: 10.3390/ijms161025234. PMID: 26512646; PMCID: PMC4632800.
- Zarei A, Changizi-Ashtiyani S, Taheri S, Ramezani M. A quick overview on some aspects of endocrinological and therapeutic effects of Berberis vulgaris L. Avicenna J Phytomed. 2015 Nov-Dec;5(6):485-97. PMID: 26693406; PMCID: PMC4678494.
- Abdou E, Hazell AS. Thiamine deficiency: an update of pathophysiologic mechanisms and future therapeutic considerations. Neurochem Res. 2015 Feb;40(2):353-61. doi: 10.1007/s11064-014-1430-z. Epub 2014 Oct 9. PMID: 25297573.
- Bhandary S, Aguan K. Pyruvate dehydrogenase complex deficiency and its relationship with epilepsy frequency--An overview. Epilepsy Res. 2015 Oct;116:40-52. doi: 10.1016/j.eplepsyres.2015.07.002. Epub 2015 Jul 8. PMID: 26354166.
- Mirmazloomi S, Shahsavani D, Baghshani H. Studies on the protective effects of ascorbic acid and thiamine on lead-induced lipid and protein oxidation as well as enzymatic alterations in some tissues of Cyprinus carpio, Comparative Clinical Pathology. 2015; 24:1231-1236.
- Cruz KJ, de Oliveira AR, Marreiro Ddo N. Antioxidant role of zinc in diabetes mellitus. World J Diabetes. 2015 Mar 15;6(2):333-7. doi: 10.4239/wjd.v6.i2.333. PMID: 25789115; PMCID: PMC4360427.
- Anwar A, Ahmed Azmi M, Siddiqui JA, Panhwar G, Shaikh F, Ariff M. Thiamine Level in Type I and Type II Diabetes Mellitus Patients: A Comparative Study Focusing on Hematological and Biochemical Evaluations. Cureus. 2020 May 8;12(5):e8027. doi: 10.7759/cureus.8027. PMID: 32528766; PMCID: PMC7282352.
- Rosner EA, Strezlecki KD, Clark JA, Lieh-Lai M. Low thiamine levels in children with type 1 diabetes and diabetic ketoacidosis: a pilot study. Pediatr Crit Care Med. 2015 Feb;16(2):114-8. doi: 10.1097/PCC.0000000000000302. PMID: 25560422.
- Riaz S. Study of Protein Biomarkers of Diabetes Mellitus Type 2 and Therapy with Vitamin B1. J Diabetes Res. 2015;2015:150176. doi: 10.1155/2015/150176. Epub 2015 Jul 27. PMID: 26273663; PMCID: PMC4530253.