More Information
Submitted: February 21, 2023 | Approved: March 30, 2023 | Published: March 31, 2023
How to cite this article: Gaspar M. Coronary artery anomalies, myocardial bridging associated with fistula to pulmonary artery trunk. Case reports. Ann Dermatol Res. 2023; 7: 009-012.
DOI: 10.29328/journal.adr.1001023
Copyright License: © 2023 Gaspar M. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Myocardial bridging; Coronary fistula
Coronary artery anomalies, myocardial bridging associated with fistula to pulmonary artery trunk. Case reports
Marian Gaspar*
University of Medicine and Pharmacy “Victor Babes”, Timisoara, Romania
*Address for Correspondence: Dr. Marian Gaspar, Professor, University of Medicine and Pharmacy “Victor Babes”, Timisoara, Romania, Email: [email protected]
Between coronary artery anomalies, myocardial bridging means an epicardial coronary artery, mostly left anterior descending artery (LAD), running through an intramyocardial “tunnel” (usually in the middle segment), leading during systolic contraction, flow reduction, through the vessel. When this anomaly is associated with a coronary fistula, which “steals” more from the bloodstream, the symptoms are more pronounced, and the management complex is surgical in particular. Despite the presence from birth remains asymptomatic and it becomes clinically manifest later in the third to fourth decade of life, with a diverse palette of symptoms; angina, arrhythmias, and acute myocardial infarction up to sudden death. Diagnosis and particular management, medical, interventional, and surgical should avoid major cardiac complications and sudden death. We present two adult patients, with coronary artery bridging, one case associated with coronary artery fistula, LAD to pulmonary artery trunk, very symptomatic with surgical management, and the second only myocardial bridging controlled with medication and supervision.
This congenital anomaly in which a coronary artery, usually left anterior descending (LAD), follows a deep path in the myocardium bridge, “tunnel”, was described morphologically several hundred years ago (Reyman,1737), but first angiographic documented, later by Portmann and Iwig in 1960 [1]. The prevalence of myocardial “bridge” at angiography is lower (0,5% – 2,5%) than at autopsy (15% – 85%). The coronary filling flow for the most part occurs in diastole, systolic compression of the artery should have only a little impact on total effective myocardial perfusion, but more refined studies using frame-by-frame quantitative coronarography with IVUS study, reveal that compression of the vessel extending also into diastole and as the result affect the myocardial perfusion [2]. Coronary bridge syndrome is also common in hypertrophic cardiomyopathy, with a frequency of 25% - 80%, and in a patient with orthotopic heart transplants 33%. Due to this association, sudden death has been described in young people and athletes. An intriguing clinical situation is a myocardial infarction in young persons, without atherosclerotic lesions discernible by coronary angiography. In the absence of atherosclerosis, myocardial infarction may result from several aetiologies including; vascular spasm, transient dysrhythmia, drug abuse, hypercoagulability and coronary thrombus formation, and dissection of acquired or congenital vascular anomalies [3]. The patients are admitted with; angina, arrhythmias, or even acute myocardial infarction with left ventricular dysfunction or worse, sudden death in young, active persons [4,5]. Another congenital anomaly is a coronary fistula, which is an anomalous communication between one or two coronary arteries and a cardiac chamber or any of the great vessels (the coronary sinus, the superior vena cava, and the pulmonary artery). Prevalence, in the general population, is 0,002%, within all congenital heart disease 0,08% – 0,4% and 0.3% – 0.8% of all patients who undergo selective coronary angiography [6]. The most common site of drainage is the right ventricle, followed by the right atrium and the pulmonary artery (PA). The blood flow from the coronary, usually LAD to PA shunt, leads to ‘coronary steal’, drawing blood away from the normal coronary tree, the results are symptoms and signs of myocardial ischemia.
Even more, if two anomalies are associated, coronary bridging and fistula, then the symptomatology is obvious and the management much more complex, medical, interventional, and surgical [7].
We do not have any specific medical therapy for coronary fistula, this should be occluded by transcatheter embolization (coils, vascular plug, covered stent) or surgical intervention (dissection and ligature of fistula on both sides).
We present two cases; the first case is a LAD bridging associated with fistulae between LAD and PA trunk, very symptomatic in spite of medical treatment, and the second only with LAD bridge, managed with medication and surveillance.
The first patient F. C, 46 years old male, presented with typical angina by exercise and at the rest, and was admitted for diagnosis and management.
ECG: Sinus rhythm, intermediate QRS axis, HR = 80 bpm.
Eco-cardiography: LV hypertrophy, FE: 55%, mild mitral regurgitation, mild tricuspid regurgitation. Normal pericardial fluid.
Chest X-ray: Heart and lungs according to age. No pulmonary condensation or fluid collections.
In spite of complex medication; Aspirin 75 mg/day, selective beta-blocker (Bisoprolol 5 mg/day), Candesartan cilexetil – (Atacand) 16 mg/day, diuretics 50/20 mg /day, ivabradine (Corlentor) 5 mg x2/day, atorvastatina (Zetovar) 40/10 mg /day, to control angina, blood hypertension, mixt-dyslipidemia, the patient continues to have exercise and rest angina. The patient was admitted for more investigation.
Angiocoronarography
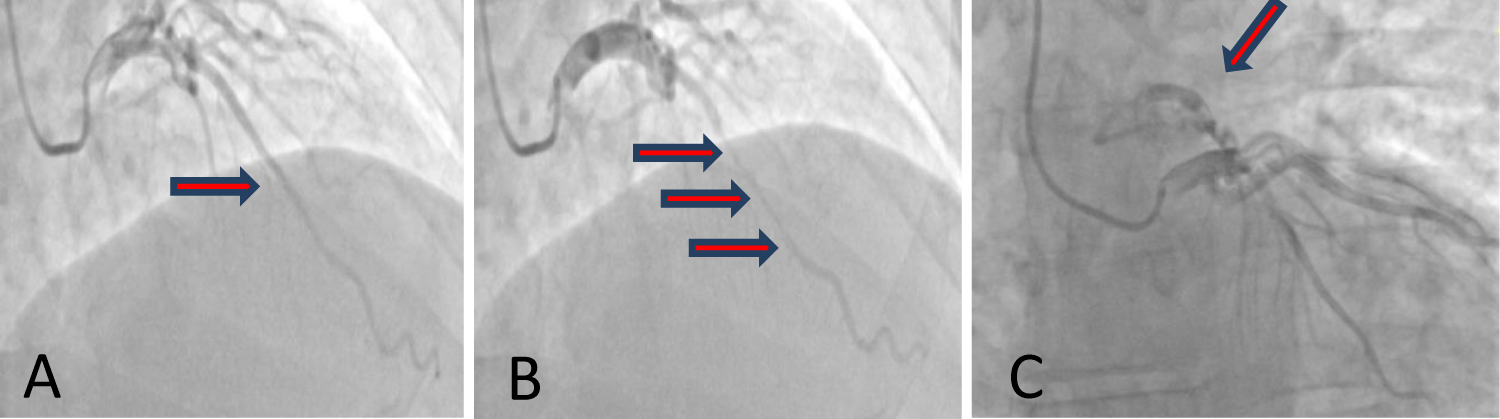
Right radial artery approach. A coronary system with right dominance. Left main, normal. Coronary arteries show marked spasm that improves with the administration of intracoronary NTG. Upon injection into the ACD, a marked negation of T waves is observed in the lower leads on the EKS. LAD presents in the middle segment, over a length of 4 cm, an accentuated muscular bridge after administration of intracoronary NTG, with 80% stenotic systolic compression. A coronary fistula emerges from the proximal ADA that drains into the pulmonary circulation. Proximal and distal LAD, circumflex artery and RCA do not present lesions visible angiographically (Figure 1).
Figure 1: Coronaro-angiography showing diastolic lumen dimensions are normal (A), typical systolic compression (red arrows) of the mid-LAD, and a long segment (B). Associated a fistula from LAD to the pulmonary artery trunk is revealed (C).
Coronary artery fistulas between the LAD and the PA are rare congenital malformations. However, concomitant significant coronary artery stenosis and fistula can cause coronary steal phenomenon and this results in severe myocardial ischemia.
In such a situation, bridging associated with coronary artery fistula, the treatment is interventional or surgical. The operation was performed through standard median sternotomy using a heart-lung machine. After the aortic was clamped, anterograde cardioplegia stopped the heart for clear inspection. First LAD was released, starting in the distal superficial segment and continuing carefully proximal dissection using a beaver, for an 8 cm segment.
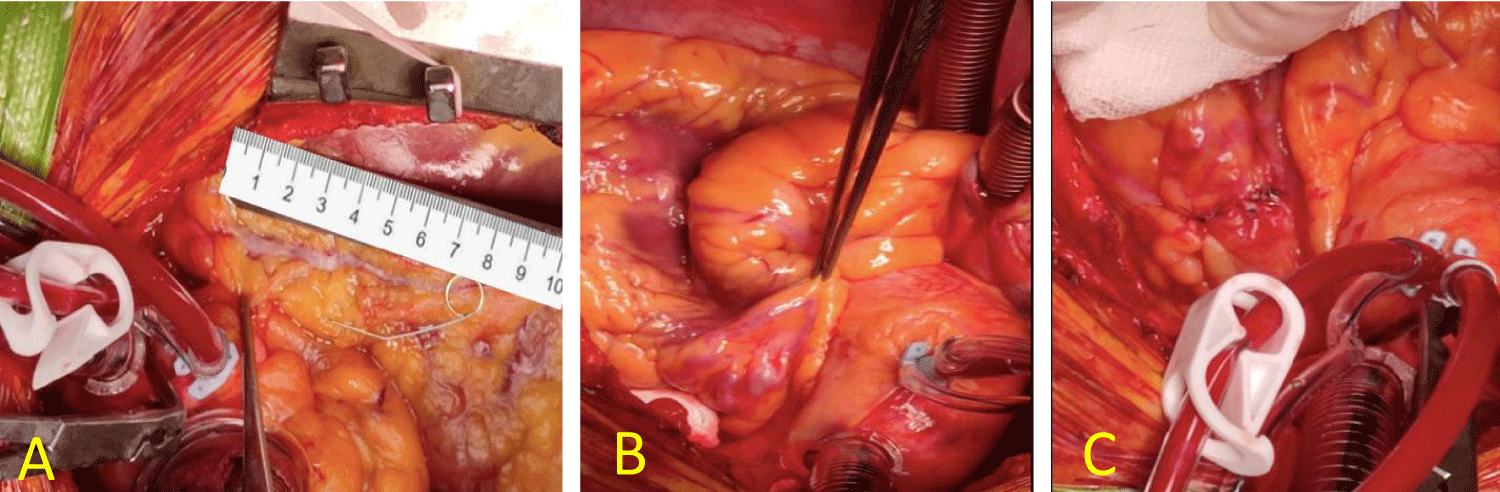
The fistula was clearly identified on the surface of the right ventricle reaching the proximal portion of the main pulmonary trunk above the pulmonary valve. After that, the fistula was tenderly dissected in special origin from LAD, then closed using two stitches of 5/0 polypropylene (Figure 2).
Figure 2: Using the heart-lung machine, the heart is stopped by anterograde cardioplegia, LAD inspected and dissected, myotomy for approximately 8 cm starting distally and carefully proximal (A), then the LAD-to-PA fistula is identified (B), isolated and sutured on both sides (C).
In the case of untimely dissection, some complications can be associated with myotomy; injury to the artery, incomplete myotomy, and right ventricular perforation. To avoid right ventriculotomy, the myotomy should be located anteriorly toward the left side of the artery.
Association of coronary by-pass, with the left internal mammary artery (LIMA) to LAD, is advised in case of proximal severe atherosclerotic stenosis, otherwise, due to competitive flow, a graft can be obstructed.
The postoperative and subsequent evolution was good, without complications and with the disappearance of symptoms.
The second patient I.G, 54 years old male, was hospitalized for precordial pain with radiation in the left shoulder, occurring after meals, upon admission, the patient was in good clinical condition, without precordial pain, without signs of heart failure with AP 115/70 mmHg, AV = 80 bpm.
ECG: Sinus rhythm, AV = 96 bpm, intermediate QRS axis, ST depression 1 mm in V6.
Cardiac echocardiography
Ascending aorta = 26 mm, aortic annulus 19 mm, LV of normal dimensions, VD = 30 mm, VS = 41 mm, SIV = 11 mm, PPLV = 10 mm, with good systolic function EF = 55%, without disturbances of parietal kinetics. LA and right cavities of normal size. Mitral insufficiency degree one, mitral flow E = 0,76. Pericardial fluid 7 mm.
Biochemistry
LDL cholesterol-135 mg/dl, HDL cholesterol = 33 mg/dl, cholesterol -204 mg/dl, Triglyceride 176 mg/dl.
Coronary angiography
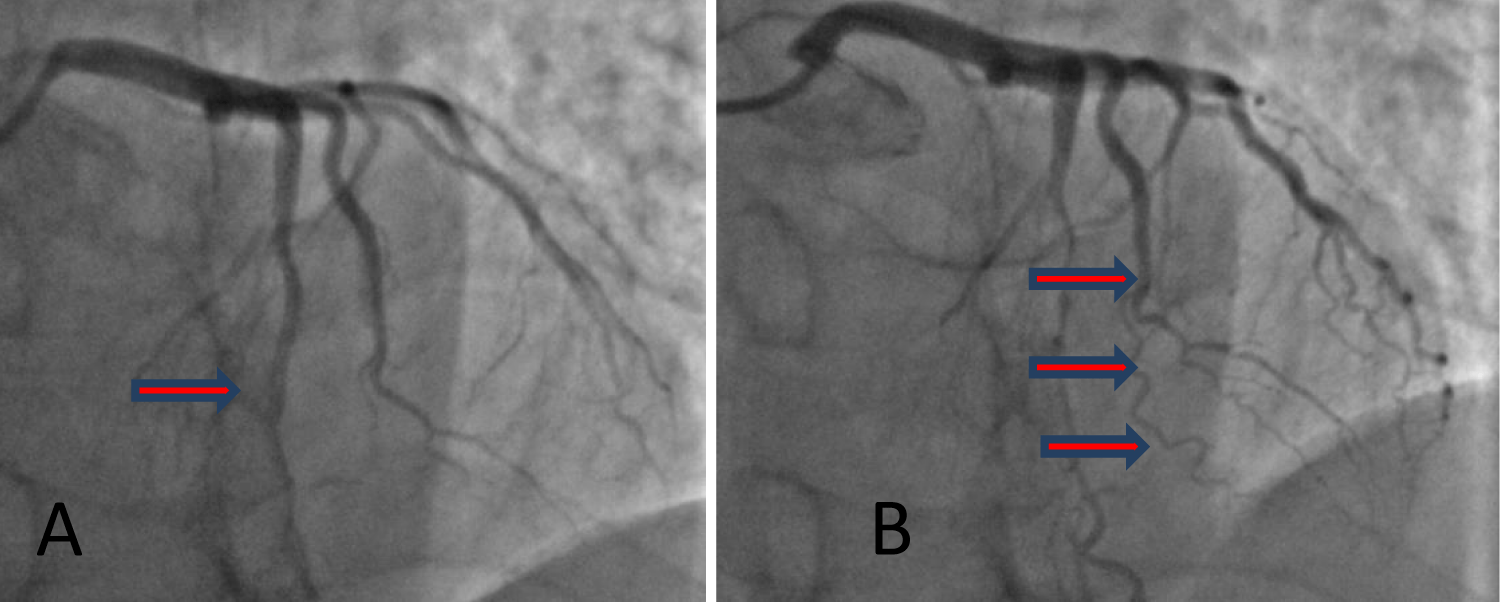
Does not show coronary stenoses on the RCA, or LCA, but shows a "muscular bridge" with LAD constriction in the middle portion, after the administration of NTG (Figure 3).
Figure 3: Coronary angiography highlights normal vessels on diastole (A) and the LAD "muscular bridge" in the middle segment, with obvious constriction when nitroglycerin is administered (B).
Management
In this case, the treatment was only medical, with B-blockers, aspirin, a combination of two lipid-lowering drugs (Leridip and Sortis), hypotensive medication (Candesartan cilexetil - Atacand, 8 mg/day) and periodic surveillance with readmission in case of angina attacks. Nitrates are contraindicated in patients with myocardial bridging. Nitroglycerin has been shown to accentuate systolic compression of bridged segments and indeed is used as an agent for the provocation of these lesions, like in our case.
Myocardial bridging of the left anterior descending (LAD) coronary artery occurs in 1% - 4.5% of coronary angiographies [8]. But the association with coronary fistula is very rare and gives it a particular, clinical, and therapeutic significance. In the case of myocardial bridging, the majority of the patients are asymptomatic. When they become symptomatic with angina, myocardial ischemia, myocardial infarction, left ventricular dysfunction, myocardial stunning, paroxysmal AV blockade, exercise-induced ventricular tachycardia, and sudden cardiac. Young patients, usually without high-risk factors for atherosclerosis (diabetes mellitus, dyslipidemia, smoking), come to the emergency department complaining of chest pain and fatigue, usually after physical exertion or stress.
Then, even if the constriction is very severe or in 90% of the cases, atherosclerotic lesions are associated, located in the proximal part of the vessel possibly due to mechanical shear stress (Haager and all) or is associated with other anomalous like in our case, coronary artery fistula, which accentuated myocardial “steal” and ischemia (first described 1865 by Krause).
Diagnosis with the new imaging techniques; cardiac echocardiography, echo-stress, coronary cine angiography, fractional flow reserve (FFR), intravascular ultrasound, (IVUS), 256 slices- Computer Tomography, has led to improved identification and functional quantitation of myocardial bridging and associated disease [9]. Coronary cine angiography remains the most used technique for diagnosing myocardial bridging.
IVUS is more sensitive than angiography (one study detected bridging in 23% of patients, while angiographic systolic compression was only apparent in 3%). The muscle tunneled segment of the artery clearly demonstrates systolic compression that persists into diastole. Also, when combined with provocation testing with nitroglycerin, dobutamine, or rapid atrial pacing.
The treatment of myocardial LAD bridging is complex, the patients are usually young and may present with severe, usually atypical, symptoms often attributed to other causes. Once the diagnosis is made, medication is considered first-line therapy, beta-blockers, calcium-channel blockers, Aspirin, and lipid-lowering agents (if the case). Volume loading may also reduce compression of the tunneled segment, whereas administration of nitroglycerine may aggravate compression and ischemia [9]. For this reason, nitrates are contraindicated in patients with myocardial bridging and indeed used as an agent for the provocation of these lesions as we did in our second case.
The interventional cardiologist (Stables, et al. 11995) first reported coronary stenting as an interventional approach to severe myocardial bridging refractory to medication but multiple cases of coronary complication, perforation and stent fracture in a stented myocardial bridge have been reported, perhaps related to stent oversizing.
In patients with the persistence of symptoms in spite of the right medication or associated coronary disease (atherosclerotic stenosis, coronary fistula), surgical manage-ment is the choice.
Surgical techniques for myocardial bridging include; myotomy (Binet 1975) associated or not with coronary artery bypass graft surgery (CABG). This is a relatively easy surgical procedure, LAD dissection, and release from the distal part to the proximal area. However, risks and complications must be taken into account, LAD damage, perforation into the right ventricle, graft occlusion in the presence of competitive flow on LIMA to LAD graft, arrhythmia, and later vessel fibrosis.
Coronary artery fistula to the great vessel and heart chambers, single or in association with others anomalous should be addressed by interventional or surgical closure [10].
The surgical approach of LAD bridging associated with the closure of the coronary artery fistula is indicated when both anomalies are associated. Dissection and ligation of the fistula, with an exploration of the pulmonary artery, is a safe and effective method of treatment of coronary artery fistula to the pulmonary artery. The myotomy is enough for LAD bridging. In the case of mild bridging symptoms, medication and surveillance can be the choice.
- Pérez-Pomares JM, de la Pompa JL, Franco D, Henderson D, Ho SY, Houyel L, Kelly RG, Sedmera D, Sheppard M, Sperling S, Thiene G, van den Hoff M, Basso C. Congenital coronary artery anomalies: a bridge from embryology to anatomy and pathophysiology--a position statement of the development, anatomy, and pathology ESC Working Group. Cardiovasc Res. 2016 Feb 1;109(2):204-16. doi: 10.1093/cvr/cvv251. Epub 2016 Jan 11. PMID: 26811390.
- Lee MS, Chen CH. Myocardial Bridging: An Up-to-Date Review. J Invasive Cardiol. 2015 Nov;27(11):521-8. Epub 2015 May 15. PMID: 25999138; PMCID: PMC4818117.
- Dwyer J. Coronary artery bridging as an etiology for non- atherosclerotic myocardial infarction: A review of literature and case history. J Cardio Case Rep. 2019. doi: 10.15761/JCCR.1000111
- Möhlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation. 2002 Nov 12;106(20):2616-22. doi: 10.1161/01.cir.0000038420.14867.7a. PMID: 12427660.
- Ripa C, Melatini MC, Olivieri F, Antonicelli R. Myocardial bridging: A 'forgotten' cause of acute coronary syndrome - a case report. Int J Angiol. 2007 Fall;16(3):115-8. doi: 10.1055/s-0031-1278262. PMID: 22477305; PMCID: PMC2733018.
- Butt K, Agha A, Parente R, Limback J, Burt JR. Anomalous Coronary Anatomy with Fistula Diagnosed on Coronary Computed Tomography Angiography. Cureus. 2019 Apr 6;11(4):e4403. doi: 10.7759/cureus.4403. PMID: 31245193; PMCID: PMC6559691.
- Kunt AS. Coronary artery and pulmonary artery fistula originated from significant stenosis in the left anterior descending artery. Case Rep Emerg Med. 2013;2013:298156. doi: 10.1155/2013/298156. Epub 2013 Feb 12. PMID: 23476823; PMCID: PMC3583081.
- Ekeke CN, Noble S, Mazzaferri E Jr, Crestanello JA. Myocardial bridging over the left anterior descending: Myotomy, bypass, or both? J Thorac Cardiovasc Surg. 2015 Apr;149(4):e57-8. doi: 10.1016/j.jtcvs.2014.12.054. Epub 2014 Dec 30. PMID: 25648479.
- Kanwal A, Shah AB. Myocardial Bridging in Adult. American College of Cardiology. https://www.acc.org/latest-in-cardiology/articles/2020/08/04/08/48/myocardial-bridging-in-adults.
- Wan L, Wu Q. Myocardial bridge, surgery or stenting? Interact Cardiovasc Thorac Surg. 2005 Dec;4(6):517-20. doi: 10.1510/icvts.2005.111930. Epub 2005 Sep 20. PMID: 17670472.