More Information
Submitted: February 25, 2023 | Approved: March 13, 2023 | Published: March 14, 2023
How to cite this article: Al-Dabbagh J, Ismail N, Ismael MH, Al-Soufi L, Al-Shehabi Z. A challenging case of cutaneous sarcoidosis with unusual findings in a Syrian woman: a case report and review of literature. Ann Dermatol Res. 2023; 7: 001-008.
DOI: 10.29328/journal.adr.1001022
Copyright License: © 2023 Al-Dabbagh J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Sarcoidosis; Cutaneous sarcoidosis; Sarcoidosis histopathology; Sarcoidosis dermoscopy; Fissured tongue
A challenging case of cutaneous sarcoidosis with unusual findings in a Syrian woman: a case report and review of literature
Jacob Al-Dabbagh1* , Nemat Ismail1, Mohammad Haitham Ismael1, Lina Al-Soufi1 and Zuheir Al-Shehabi2
, Nemat Ismail1, Mohammad Haitham Ismael1, Lina Al-Soufi1 and Zuheir Al-Shehabi2
1Department of Dermatology, National Hospital of Latakia, Latakia, Syria
2Department of Pathology, Tishreen University Hospital, Latakia, Syria
*Address for Correspondence: Jacob Al-Dabbagh, Department of Dermatology, National Hospital of Latakia, Latakia, Syria, Email: [email protected]
Sarcoidosis is a granulomatous disease that involves multiple systems. Cutaneous involvement can manifest in patients with sarcoidosis and can present with or without systemic involvement.
We present a case of cutaneous sarcoidosis in a Syrian woman that showed improvement after a combination of methotrexate and prednisolone therapy. The patient had unusual chest radiography findings and developed an unexplained fissured tongue after 5 days of receiving methotrexate therapy. In addition, the patient developed indurated erythematous plaque and papules on her upper right arm at the same location as a performed biopsy, which increased the diagnosis of a newly formed scar sarcoidosis although she was receiving her treatment.
Sarcoidosis is a chronic inflammatory disorder of unknown etiology that can affect multiple organs [1]. The lungs and lymph nodes (90%), eyes (40%) and skin (25%) are the most involved organs in sarcoidosis [2]. Sarcoidosis can affect patients of any age, race, ethnicity, and sex/gender [3]. It is more common in adults and reaches two peak incidences [1,4]. The first peak incidence is in the third to fourth decade of age and the second is at the ages of 65 years – 69 years [1,4]. The highest incidence is reported in African-Americans and the white population, while the lowest incidence is observed in Asians and Hispanics [5]. The prevalence of sarcoidosis is higher in females, rustic communities and non-smokers [3,4]. Females have a slightly higher risk compared to males [1]. Other multisystemic granulomatous disorders must be excluded to confirm the diagnosis of sarcoidosis due to the clinical presentation of sarcoidosis that can mimic other various diseases [5]. The presence of non-caseating granulomas in biopsy specimens can support the diagnosis, but it is not adequate to make a distinct diagnosis [5].
A 60-year-old Syrian woman presented with violaceous and red erythematous lesions localized on her nose, philtrum and chin. The lesion, which was located on her nose, started 7 years ago as an erythematous plaque that enlarged gradually and became rhinophymatous. Then it extended to the philtrum. Other erythematous plaques appeared on her chin, upper right arm, right forearm and left lateral thigh. There were no signs of inflammation, pruritus, or pain.
Four months after the lesions appeared, the initial diagnosis of rhinophyma was made by a physician, and the patient was treated with oral isotretinoin, without improvement. Also, the diagnosis of leishmaniasis was suspected by the same physician. Hence, a parasitological smear was performed and was negative.
Five months later, the diagnosis of sarcoidosis was suspected by another physician. Thus, it was decided to introduce topical steroids associated with oral prednisolone (20 mg/day), with no response to the therapy.
Then the patient was treated with antimalarial courses. In addition to receiving 2 doses of intracutaneous injections of triamcinolone. However, there was no improvement either.
It is worth mentioning that the patient underwent many tests and examinations, including a chest X-ray. All the tests were normal and no extracutaneous involvement was detected.
After a few months, she visited another physician who also diagnosed her with rhinophyma and treated her with doxycycline. However, treatment with doxycycline did not lead to any improvement. Then she was treated with oral prednisolone (60 mg/day) due to a suspected immune disease. The treatment with prednisolone showed a slight improvement, then it was withdrawn due to its high toxicity.
Anyway, the patient stopped receiving treatment before the last two years of her visit to our department.
None of the previous physicians confirmed any of the above-mentioned diagnoses by obtaining a skin biopsy.
There was no recent history of fever or loss of the patient’s weight. Also, she has no previous history of allergic reactions to any component.
Her family history regarding inherited skin disorders was unremarkable. She did not recall any insect bites before the lesions appeared.
The patient was a former smoker. She started smoking at the age of 35 and quit smoking at the age of 42.
Her past medical history included: Hypothyroidism, which was developed in her early twenties and treated with thyroxine. High blood pressure, which was developed 15 years ago and was treated with bisoprolol and losartan. Knee pain, which is associated with activities and prolonged weight-bearing, did not receive any specific treatment. A non-explained productive cough.
In addition, she had cataract surgery on her right eye 15 years ago and on her left eye 3 years ago.
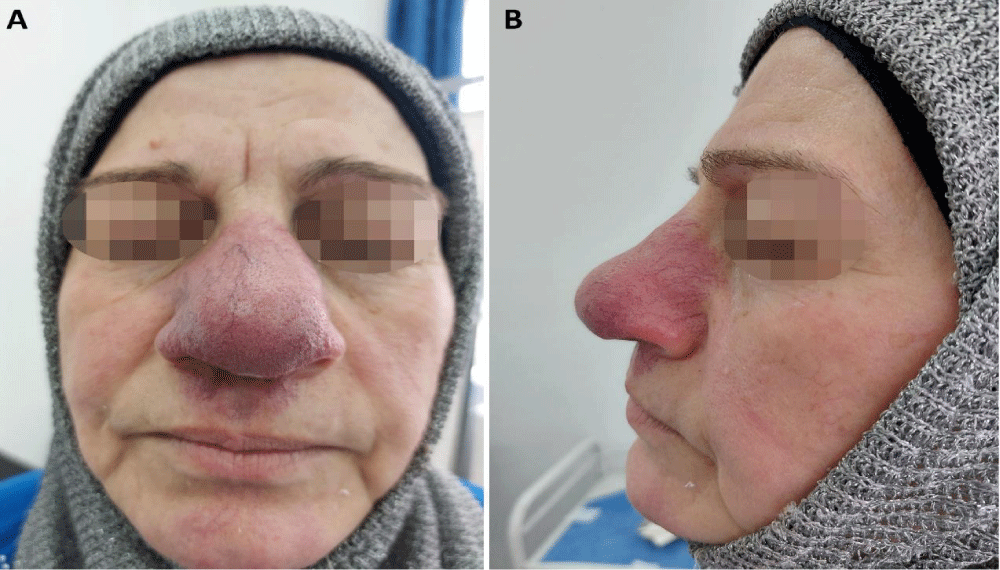
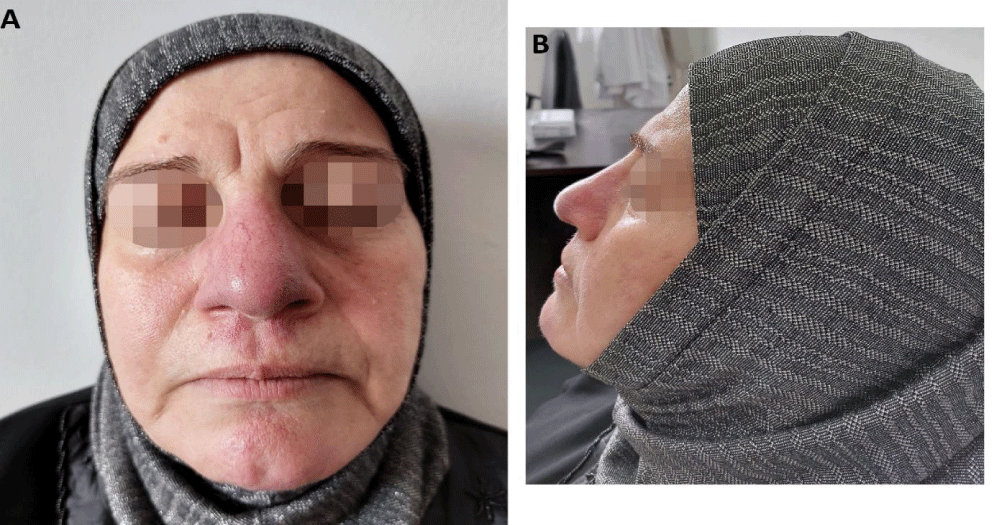
The dermatologic examination revealed painless indurated erythematous plaques with irregular borders in the same above-mentioned locations. Also, telangiectasias were noted on her nose and philtrum, which developed, according to the patient, after receiving the intracutaneous injection of triamcinolone (Figure 1).
Figure 1: Cutaneous sarcoidosis: Plaques affecting the nose and philtrum with progressive enlargement of the nose, and facial telangiectasias. A: Antero-posterior view, B: Lateral view.
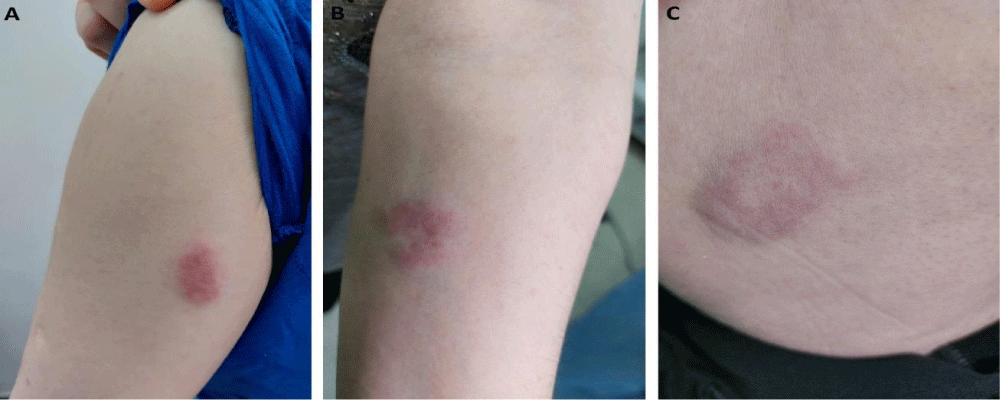
The measures of the plaques on the limbs were as follows: 3.5 x 1.4 cm on the left upper arm, 2.5 x 3.1 cm on the left forearm and 4.3 x 3.7 cm on the lateral thigh (Figure 2).
Figure 2: Cutaneous sarcoidosis: Plaques affecting the left upper arm (A), the left forearm (B) and the lateral thigh (C).
No mucosal abnormalities were noted. The physical examination was otherwise normal. There was no fever, palpable lymph nodes, or organomegaly.
We considered many differential diagnoses, which include cutaneous sarcoidosis, cutaneous leishmaniasis, cutaneous tuberculosis, lupus erythematosus and rhinophyma.
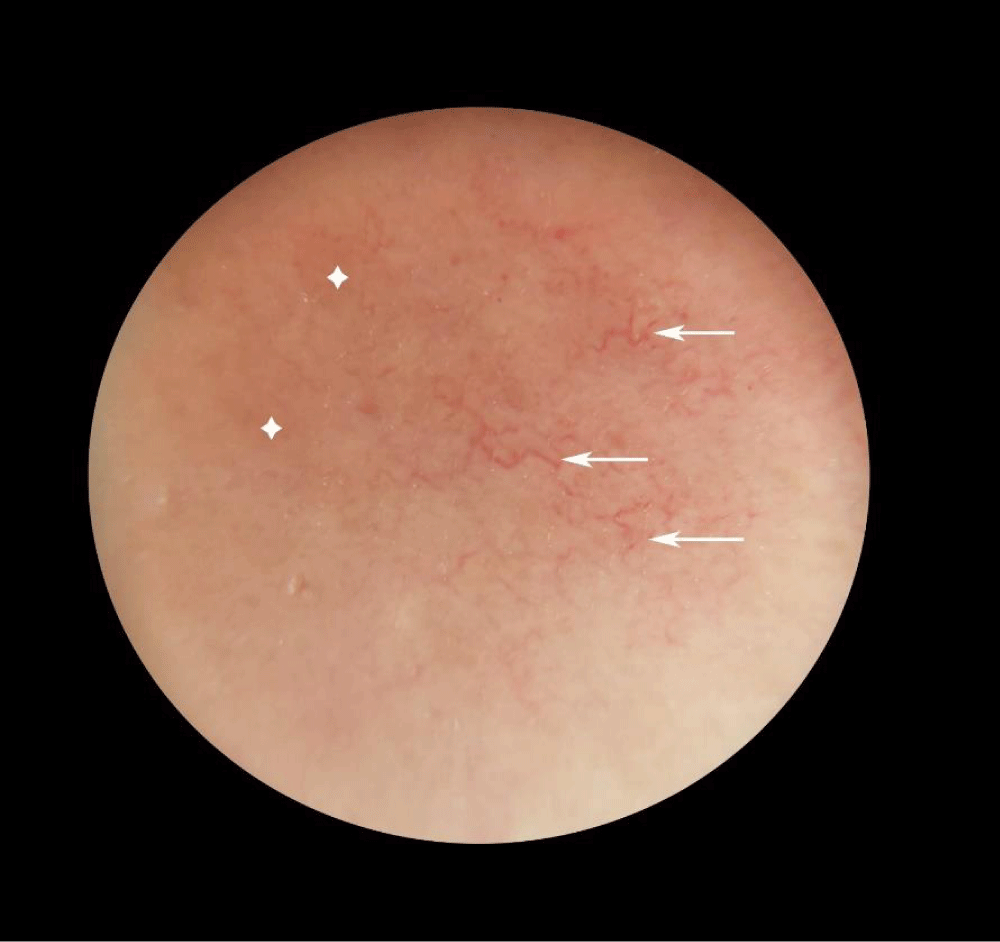
Dermoscopy was performed and revealed yellowish-orange structureless areas with branching vessels in the plaque that was located on the upper arm (Figure 3).Figure 3: The dermoscopic examination revealed: Multiple linear and branching vessels (arrows) with yellowish-orange structureless areas (stars).
Based on the dermoscopic findings, the possibility of dermal granulomatous infiltration was considered. Therefore, cutaneous sarcoidosis, leishmaniasis and tuberculosis were at the top of our differential diagnoses list.
Two biopsies were performed on the edge of the philtrum and upper arm plaques.
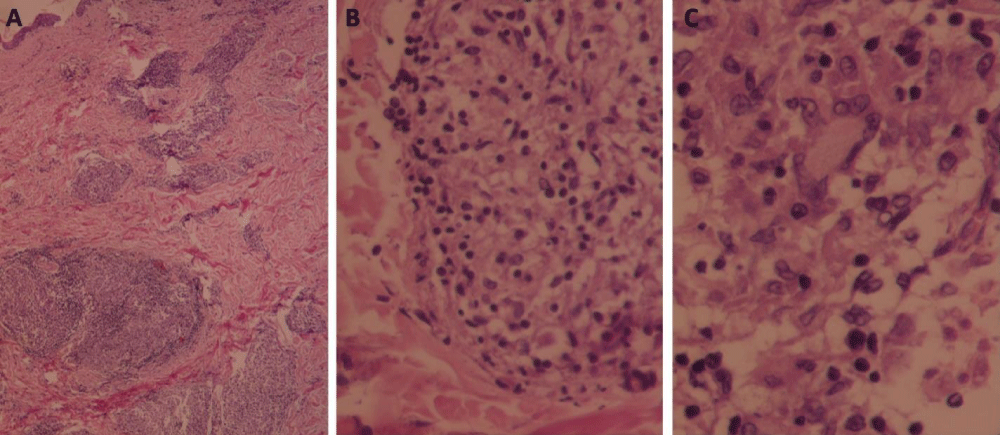
The histopathologic features demonstrated the following macroscopic description: Mild epidermal atrophy, focal erosion, parakeratosis, a few granulomatous aggregates without central necrosis occupying the reticular and deep dermis; consisting of occasional epithelioid cells, a few multinucleated giant cells of Langhans' type and foamy histiocytes and rare lymphocytes at the periphery; surrounded by scanty fibrous connective tissue and the proliferation of thick-walled vessels. No cellular atypia was noted within the limits of the examined biopsy (Figure 4).
Figure 4: Cutaneous sarcoidosis: A. A few granulomatous aggregates occupy the reticular and deep dermis (H&E, x100). B. The granuloma consists of occasional epithelioid cells, foamy histiocytes, and rare lymphocytes at the periphery (H&E, x200). C. A few multinucleated giant cells of Langhans' type (H&E, x400).
The results of the routine laboratory tests, including complete blood counts (CBC), AST, ALT, CRP, LDH, TSH, K, Na, Ca, creatinine, glucose and urea, were within the normal limits except for the 25-hydroxy vitamin D3 rate, which was 9.6 ng/ml (the expected values are 30 – 90 ng/ml). Urinalysis was normal. The ECG showed a normal pattern.
An Angiotensin-Converting Enzyme (ACE) test was considered to support the diagnosis. But it was not available in our hospital.
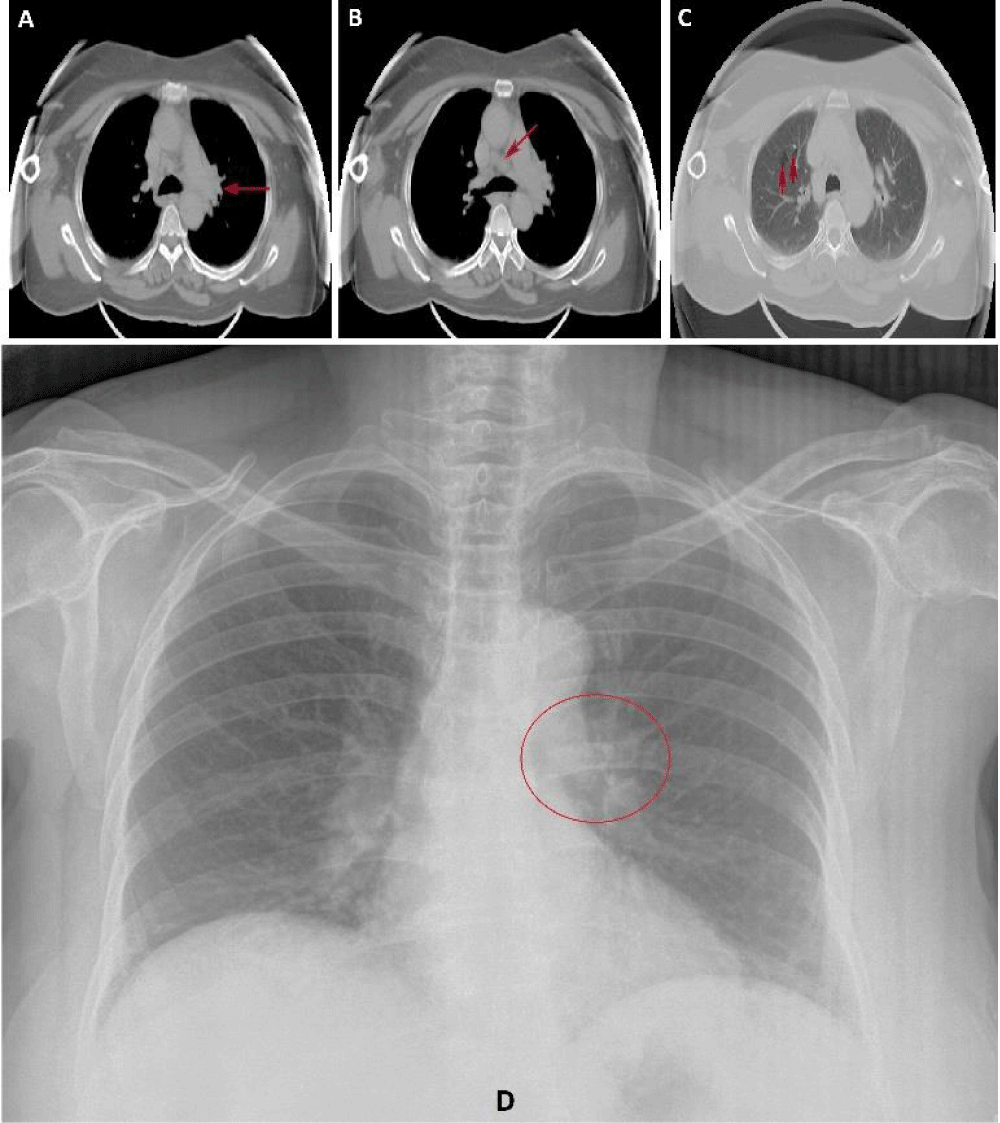
CXR shows unilateral left hilar lymphadenopathy (Figure 5).
Figure 5: Chest CT scan shows an increase in the size of the left hilar lymph node (A, arrow) and mediastinal lymph node (B, arrow). Lung bullae in the right lung (C, arrows). Chest X-ray demonstrating unilateral left hilar lymphadenopathy (D, circle).
Sonography of cervical, axillary and inguinal lymph nodes was performed and did not reveal any abnormalities. Sonography of supraclavicular and infraclavicular lymph nodes was not performed at this stage.
Transverse gray-scale sonography of the thyroid gland shows diffuse enlargement of the thyroid gland with multiple small and well-defined hypoechoic nodules involving both the lobes and the isthmus. The largest nodule was 1 cm in size and located in the left thyroid lobe. The thickness of the thyroid isthmus was 8 mm.
The sonography also shows a 6 mm, well-defined, round nodule at the junction of the left lobe of the thyroid.
As Syria is one of the countries where leishmaniasis is prevalent, the diagnosis of lupoid cutaneous leishmaniasis was suspected in our patient. Thus, a parasitological smear after skin scraping was obtained from the lesions. The microscopic examination did not find any parasites.
A tuberculin skin test was performed and was negative. In addition, sputum was smear-negative for acid-fast bacilli.
The diagnosis of sarcoidosis was established based on the patient’s tests, together with the histopathologic and clinical findings.
The patient was referred to the respiratory, cardiology, endocrinology, rheumatology, ophthalmology and radiology departments for further investigation.
Cardiovascular and ocular examinations were normal. The patient was diagnosed with osteoarthritis by a rheumatologist based on the patient’s examination and knee X-ray.
The patient did not complain of any respiratory symptoms except for a recurrent cough, which first appeared in childhood as a dry cough, then developed into a productive cough with white-colored sputum. Chest auscultation and pulmonary function testing were normal.
A chest CT scan confirmed the presence of unilateral left hilar lymphadenopathy. It also showed mediastinal lymph node enlargement (more than 1 cm) and lung bullae, which were more common in the right lung (Figure 5).
The thyroid fine needle aspiration biopsy and bronchoscopy with transbronchial biopsy were recommended by physicians to obtain a suitable diagnosis based on the radiological findings in the chest CT scan and the thyroid ultrasound, but these procedures were refused by the patient as they were considered to be part of an invasive diagnostic workup.
Since the patient had received many previous treatments for sarcoidosis and there was no response except for the high dosage of prednisolone treatment, we decided to treat her with methotrexate in addition to prednisolone with a lower dosage.
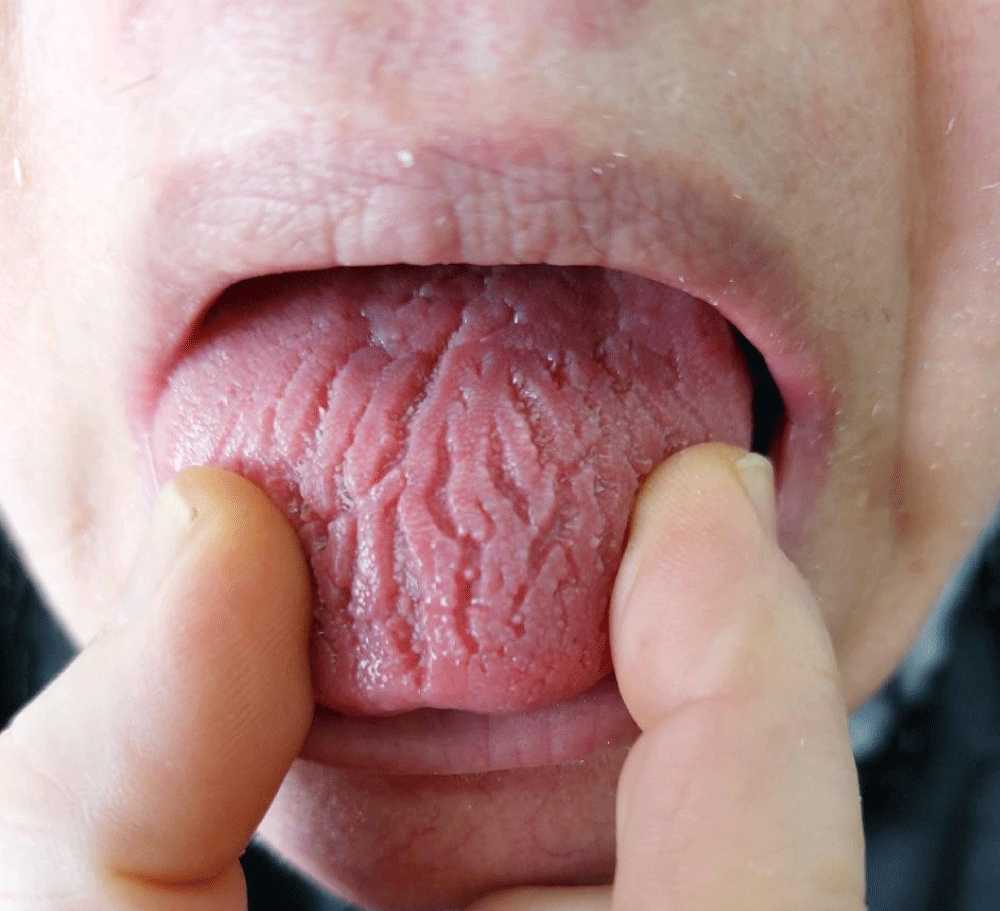
Oral prednisolone (20 mg/day) and methotrexate (10 mg/week) treatments were started. In addition to folic acid (5 mg/day) to reduce the adverse side effects of methotrexate. After 5 days of methotrexate treatment, the patient complained of discomfort, pain and a burning sensation in her tongue during the accumulation of food on it. Oral mucosal examination revealed a fissured tongue (Figure 6).
Figure 6: Fissured tongue (the cracks are widely distributed and obvious).
We could not find any explanation in the literature for methotrexate, prednisolone, or sarcoidosis as a cause of a fissured tongue. The patient does not have any systemic symptoms of deficiency of B vitamins. In addition to that, she gets a wide variety of sources of food.
The laboratory tests, including complete blood counts (CBC), AST, ALT, creatinine, glucose and urea, were repeated and were all within the normal limits except for neutrophils, which was 75.5% (the expected absolute value of 37.2% - 70%) and lymphocytes, which was 19.2% (the expected absolute value of 21.9% - 52.6%).
The treatment was continued for another 30 days with a gradual reduction of oral prednisolone dosage (10 mg/day) and an increase in methotrexate dosage (15 mg/week).
Her nose showed cosmetic results with a decrease in its size after 2 months of therapy except for the telangiectasias which were located on her nose and philtrum (Figure 7).
Figure 7: A significant regression of the lesion and the size of the nose. A: Antero-posterior view, B: Lateral view.
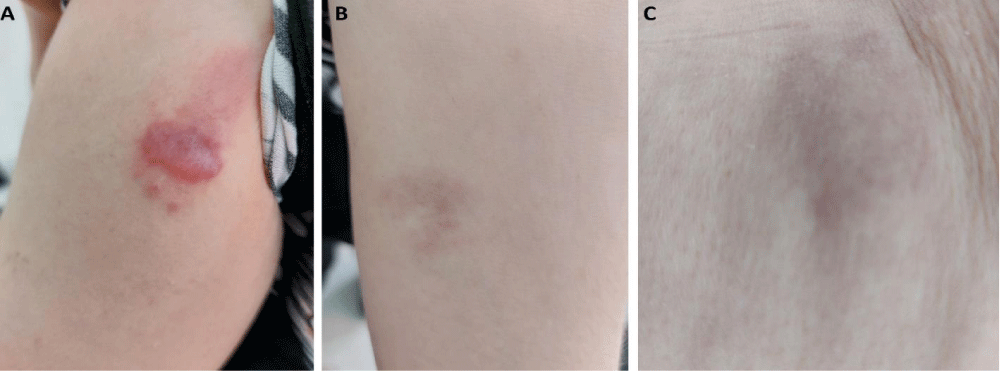
The cutaneous lesions that were located on the limbs showed marked improvement without any change in their size. Nevertheless, the patient developed infiltrative erythematous plaque and papules on her upper right arm at the same location as the performed biopsy, which increased the diagnosis of a newly formed scar sarcoidosis (Figure 8).
Figure 8: Regression of cutaneous sarcoidosis without changing the size of the lesions on the left forearm (B) and the lateral thigh (C). except for a newly developed lesion at the same location as the performed biopsy (A).
The laboratory tests were repeated, and they included complete blood counts (CBC), AST, ALT, creatinine, glucose and urea. They were all within the normal limits except for neutrophils, which were slightly elevated at 71.5% (the expected absolute value of 37.2% - 70%), lymphocytes, which were at 18.4% (the expected absolute value of 21.9% - 52.6%) and eosinophils, which was 10.1% (the expected absolute value of 0.4% - 7.5%). The bone density test was performed and was within the normal score.
The follow-up chest CT scan, 2 months after the first performed one, did not show any improvement compared to the formerly observed findings, except for a decrease in the mediastinal lymph node (7.8 mm).
Sonography of cervical, axillary, inguinal, supraclavicular and infraclavicular lymph nodes was performed and did not reveal any abnormalities.
The patient was satisfied with the treatment results and she still followed up and monitored regularly. Also, the cough was soothed with a reduction in its frequency after 2 months of reviving her treatment.
We advised the patient to undergo laser therapy to decrease the likelihood of telangiectasias and obtain more aesthetic results.
Sarcoidosis is a multisystemic inflammatory disorder of unknown etiology, in which non-caseating granulomas are present in many affected organs [6]. The most common involvements include lungs, lymph nodes and skin [6]. The skin is the most common organ affected by sarcoidosis, following the lungs and lymph nodes [5].
Cutaneous sarcoidosis is divided into two types which are almost equally common: Specific skin lesions (defined by granulomatous infiltrating in the skin) and non-specific skin lesions (resulting from a systemic immunologic reaction) [1,5]. Macules, papules and plaques with yellow-brown, erythematous and violaceous colors are the most common manifestation in specific skin lesions [1,5]. In addition, specific skin lesions can present as lupus pernio, which is characterized as midfacial plaques [1].
Plaque sarcoidosis usually exists in round, oval, or annular shapes with a color variation of red-brown, purple-brown and flesh-colored [2,3]. The most involved locations are the extremities, buttocks and face [2,3]. Plaque sarcoidosis often heals with constant scarring or changes in its pigmentation [2]. It is usually associated with chronic systemic disorders, such as pulmonary and lymphadenopathy disorders [2].
Besides, Lupus pernio manifests as smooth violaceous, telangiectatic, erythematous, indurated plaques located on the central face, precisely on the nose, forehead, cheeks and lips, in addition to the ears [2,3,6]. Based on the clinical presentation of our patient the diagnosis of plaque sarcoidosis and lupus pernio as two subtypes were suggested. Lupus pernio tends to affect women and African-Americans, and it often requires aggressive systemic therapy due to the difficulty of this subtype of treatment [2,3,6]. If lupus pernio is not treated, it can corrode the underlying cartilage and bone and cause extensive destruction and disfigurement [2]. Due to the association of lupus pernio with upper respiratory or nasal mucosa involvement, granulomatous infiltration can lead to ulcerations, masses, or lethal airway obstruction [2].
Since the patient's nose was involved with cutaneous sarcoidosis, there were many considerations regarding the location of the nose and its importance from an aesthetic point of view [7]. The nose is the central part of the middle face and has an essential aesthetic, functional, and psychological role [7].
Many differential diagnoses were considered before confirming the diagnosis of sarcoidosis based on our patient’s case. The lesion on our patient’s nose mimics rhinophyma due to the clinical features of rhinophyma that were common in our patient. Rhinophyma is characterized by erythema and telangiectasias with progressive enlargement of the nose [7,8]. The diagnosis of Lupus erythematosus was also considered due to its varied expression of the clinical presentation, which can include erythema and telangiectasias [9].
The possibility of diagnosing granulomatous diseases was increased based on the dermoscopy finding. Dermoscopy is a helpful non‑invasive tool that can assist in reaching the diagnosis of granulomatous diseases [10]. The presence of orangish‑yellow structureless areas and an orange background along with linear or branching vessels support the diagnosis of granulomatous diseases [10]. The dermoscopic findings in our case correspond to the dermoscopic findings of cutaneous granulomatous disorders. Therefore, cutaneous sarcoidosis, cutaneous leishmaniasis and lupus vulgaris were considered the most probable differential diagnoses.
Cutaneous Leishmaniasis (CL) was easily suspicious as a differential diagnosis due to the frequent incidences of CL in Syria, especially in the western part of the country where the Latakia governorate was located (the patient’s hometown) [11]. Furthermore, the diagnosis of CL in our patient was supported due to the clinical features that correspond to CL like longstanding, non-itchy, painless, erythematous and infiltrated plaques that did not respond to previous systemic treatments and can be located on uncovered body areas [12,13].
Lupus vulgaris, a chronic form of cutaneous tuberculosis, should also be considered regarding the diverse clinical manifestations involving patches and plaques that are localized to the face, including the nose and cheeks [14,15]. Furthermore, Lupus Vulgaris is commonly seen in women [14,15].
Our patient underwent several tests including Acid-Fast Bacillus (AFB) and Tuberculin Skin Test (TST) which were negative. Therefore, the diagnosis of tuberculosis was excluded. Also, the diagnosis of leishmaniasis was eliminated due to the negative result of the slit skin smear.
The diagnosis of cutaneous sarcoidosis can be challenging due to lesional polymorphism [4]. Clinical features of plaque sarcoidosis can share similar features in other cutaneous disorders; therefore, another differential diagnosis may be considered [2]. The diagnosis of cutaneous sarcoidosis becomes more possible after the exclusion of the other differential diagnoses of granulomatous inflammation based on the histopathologic study along with the accordant clinical presentation [4,5].
Incisional or punch biopsy is more recommended compared to superficial shave biopsy due to the possibility of granulomatous involvement within the dermis or in subcutis tissue [3]. In our case, two incisional biopsies were obtained from two different sites.
The characteristic histopathologic features of sarcoidosis include naked or non-caseating granulomas, which are organized clusters of epithelioid cells and multinucleated giant cells and they are often surrounded by lymphocytes [2,5]. The histopathologic differential diagnosis of sarcoidosis may include many diseases such as tuberculosis and leishmaniasis [2], which were also considered based on our case.
In our case, the diagnosis of sarcoidosis is confirmed based on the histopathologic examination and accordant clinical presentation of the patient. In addition, the exclusion of other diseases that cause granulomatous inflammation.
Once the diagnosis of sarcoidosis is established, a systematic evaluation should contain physical examination including ophthalmologic examination, laboratory analysis (complete blood count, calcium, creatinine levels, angiotensin-converting enzyme, vitamin D, liver function tests, and urinalysis), ECG and chest radiography [2,5,16].
Angiotensin-Converting Enzyme (ACE) test is positive only in 60% of patients with sarcoidosis, it is non-specific for confirming the diagnosis due to its poor sensitivity and false negative result [2,16]. Therefore, it cannot be considered an essential diagnostic test [2,16]. Anyway, this test was not performed due to unavailability.
Systemic symptoms such as fatigue, fever and weight reduction can arise in one-third of sarcoidosis patients [1]. Nonetheless, the patient did not have any of the mentioned symptoms.
The lungs and thoracic lymph nodes are the most frequently affected organs in sarcoidosis (over 90% of cases) [1]. Other extracutaneous organs are less affected, such as the ocular system (more than 40%), cardiac involvement (20% – 27%), liver and spleen involvement (18%), bones involvement (1% – 13%), nervous system (less than 10%), urinary system (5%) [4]. In addition, thyroid dysfunction is seen in up to 5% of patients [4].
Pulmonary sarcoidosis is often diagnosed when radiographic abnormalities are determined during the routine examination [4]. Based on the occurrence of lymphadenopathy and/or infiltration of the lungs, pulmonary sarcoidosis can be classified into IV stages [4,5]. CT scan is more sensitive and specified than a chest X-ray for distinguishing many pulmonary complications [17].
The classical intrathoracic findings of pulmonary sarcoidosis include bilateral hilar lymphadenopathy, media-stinal lymphadenopathy, parenchymal infiltration, and predominantly in the middle and upper lobes [18]. Anyway, the radiological findings of our patient include unilateral left hilar lymphadenopathy, mediastinal lymph node enlargement and lung bullae. We could not perform any further procedures such as bronchoscopy with transbronchial biopsy due to the patient's refusal of any invasive diagnostic techniques.
Lymphadenopathy is the most common radiographic ab-normality of pulmonary sarcoidosis that occurs approximately in 80% of patients [18,19]. Bilateral hilar lymphadenopathy is the commonly observed finding with a predominance on the right side [18,19]. Moreover, unilateral hilar adenopathy occurs only in 3% – 5% of patients [18].
The common symptoms of pulmonary sarcoidosis include dry cough, dyspnea, wheezing, and chest tightness [5]. Otherwise, the patient did not have any pulmonary symp-toms except for a productive cough. Almost 50% of patients who are with pulmonary sarcoidosis are asymptomatic, particularly patients with stage I [5]. There is a delay in diagnosing patients who have pulmonary symptoms more than patients who present with cutaneous sarcoidosis [17]. Presumably, pulmonary symptoms are non-specific and could be mistaken for other more common pulmonary disease manifestations [17].
The general prognosis of pulmonary sarcoidosis is good [5]. The spontaneous regression of radiographic abnormalities is noticed in up to 80% of sarcoidosis patients with stage I [5]. In contrast, the prognosis of chronic respiratory impairment has a very low rate (less than 5%) over a 10-year period [5].
Along with the skin involvement and the pulmonary findings, our patient also had unusual thyroidal findings based on the ultrasound scan. A thyroid fine needle aspiration biopsy was recommended to obtain a proper diagnosis. Anyhow, the procedure was also refused by the patient. The past medical history of our patient included hypothyroidism. Some reports suggested that autoimmune thyroid diseases such as hypothyroidism, hyperthyroidism and Graves’ hypothyroidism are associated with sarcoidosis [20,21].
The patient underwent all the therapeutic options for sarcoidosis except for methotrexate, azathioprine and biological agents. In the first month of treatment, the patient received prednisolone (20 mg/day) and methotrexate (10 mg/week) along with folic acid (5 mg/day) therapy. In the second month, the dosage of prednisolone was reduced (10 mg/day). In return, the dosage of methotrexate was increased (15 mg/week) to decrease the possibility of prednisolone toxicity.
The treatment decision for sarcoidosis is based on the evolution of specific symptoms and sarcoidosis advancement evidenced by the worsening functional situation and radiographic abnormalities [4]. Not every patient with sarcoidosis needs to be treated [4]. Therapeutic procedures should include the mental, emotional, and physical health of the patient [4].
There are many therapeutic options regarding the extent of involvement, clinical symptoms and presentation of cutaneous sarcoidosis [1]. Therapeutic options can be categorized into local therapies (topical and intralesional corticosteroids) and systemic therapies (immunomodulatory and immunosuppressive the-rapy) [1]. Topical corticosteroids, intralesional corticosteroids, tetracycline and steroid-sparing topical agents are the first-line therapy for cutaneous sarcoidosis [3]. If the first-line therapy did not show any efficiency for mild and more extensive diseases, then second-line therapy should be considered [1]. Second-line therapy includes hydroxychloroquine, azathioprine and methotrexate [16]. Oral glucocorticoids could be considered first-line therapy for patients with rapidly developed or extensive cutaneous sarcoidosis [5]. Anyway, oral glucocorticoids are considered to be the second-line therapy after the failure of the first-line therapy [5].
Biologic agents (such as Infliximab, Adalimumab and Etanercept) are strongly recommended when the above-mentioned treatment plans fail [5].
Intralesional corticosteroids can be injected into cutaneous sarcoidosis [1]. The side effects may include atrophy, hypo-pigmentation, striae and the formation of telangiectasia [1]. Anyhow, our patient developed telangiectasias after the intracutaneous injection of triamcinolone.
The amalgamation of corticosteroid and methotrexate as a planned therapy for sarcoidosis has an important role to reduce the corticosteroid dose [16]. Some cases of sarcoidosis endure resistance to both corticosteroid and corticosteroid-sparing agents, therefore alternative therapeutic options have to be considered [6].
Corticosteroid is the backbone of pulmonary sarcoidosis treatment [17]. whereas immunomodulatory, immuno-suppressive and biologic agents are extensively used in patients who cannot tolerate or fail to respond to corticosteroid treatment [17]. The addition of methotrexate to glucocorticoids could be recommended to improve pulmonary status [22].
Methotrexate is the most frequently used for pulmonary sarcoidosis [5]. The typical dose is between 10 and 25 mg per week [5]. Anyhow, methotrexate has a slow commencement of response [5]. Its utmost efficacy will not be noted until at least 2 to 3 months after receiving the therapy [5].
The recommended dose of prednisolone is 0.5 - 0.75 mg/kg, which may be too high [4]. Wherefore, 20 mg of prednisolone can be prescribed instead [4]. The therapy can terminate after the improvement of pulmonary function [4].
To decrease the adverse effects of methotrexate treatment the co-administration of folic acid is recommended with doses ranging from 1 to 5 mg per day [23].
The major side effects of prednisolone include hypertension, osteoporosis, diabetes and weight gain [22]. The monitoring procedures of prednisolone are blood pressure, bone density and serum glucose [22].
Methotrexate has been associated with major toxicity, including leukopenia, hepatotoxicity and nausea [22]. The routine monitoring of methotrexate includes CBC, hepatic and renal serum testing [22].
After 5 days of methotrexate treatment, the patient developed a fissured tongue. Fissured tongue is a condition affecting the dorsum of the tongue as a deep groove [24]. The etiology of the fissured tongue is unknown and is classified into congenital and acquired types [24]. Trauma, syphilis, pustular psoriasis, benign migratory glossitis, malignancy and nutritional Deficiency (folic acid, biotin, riboflavin) consider possible causes of the acquired fissured tongue [24]. The fissured tongue does not require any specific treatment [24]. The general recommendations should include keeping oral hygiene, flossing, and hydration [24].
We could not find any relation between the fissured tongue and sarcoidosis or treatment with methotrexate or prednisolone during our search in the literature. It is noteworthy that Mucosal lesions of sarcoidosis are rare to be reported [2]. There are various types of lesion patterns including ulcers, edema, papules, papulonodules, localized swelling, gingival recession gingivitis, or gingival hyperplasia [2].
After 2 months of starting the treatment, the patient shows a remarkable improvement, except for the presentence of telangiectasias.
Modern laser treatment such as IPL, PDL and long-pulsed KTP-Nd: YAG laser has emerged as the first-line treatment for telangiectasias located on the face and achieved appropriate results [7].
We also observed an arising of plaque and papules on the biopsy scar of the patient’s arm. The most likely diagnosis is a new formation of cutaneous sarcoidosis on the scar. Cutaneous sarcoidosis has been reported to occur in scar tissue and can arise within surgical incisions, tattoos, and traumatized sites [2,3]. It can also develop in old and chronic scars [2,3]. The scar with violaceous or erythematosus color may specify the reactivation of the underlying process of sarcoidosis [2]. Anyway, a slight improvement of the arising plaque and papules was observed in the last visit of the patient to our department. Since the patient is still receiving her treatment which affects scar sarcoidosis as well, the diagnosis of scar sarcoidosis was strongly supported.
The oral mucosa and the tongue were examined too in her last visit without showing any improvement in the fissured tongue.
The diagnosis of our patient posed a significant challenge due to the clinical manifestations of the lesions that can mimic other skin diseases. Besides, the patient presented with unilateral hilar lymphadenopathy and productive cough which are unusual findings in sarcoidosis patients.
We reported the amalgamation of corticosteroid and methotrexate as an effective treatment plan after the patient received previous treatment plans consisting of the first and second lines of sarcoidosis treatment with no response, except for the high-dose of prednisolone that showed a slight improvement.
Although the received treatment, our patient developed indurated erythematous plaque and papules on her upper right arm at the same location as the biopsy, raising the possibility of a newly formed scar sarcoidosis.
In addition, the patient developed an unexplained fissured tongue after 5 days of methotrexate treatment. We would like to point out whether there might be an association between sarcoidosis, treated with methotrexate, and fissured tongue as they consider disorders of unknown etiology.
Informed consent: Informed, written consent was received from the patient for whom photographs are present in the manuscript.
Author contributions
Jacob Al-Dabbagh: Wrote and edited the manuscript, performed the literature review and critically revised the manuscript. Nemat Ismail: Collected the patient’s data, performed the literature review and participated in revising the manuscript. Mohammad Haitham Ismael and Lina Al-Soufi: The supervisors. Zuheir Al-Shehabi: The guarantor and performed the pathological examination. All authors read and approved the final manuscript.
- Wanat KA, Rosenbach M. A practical approach to cutaneous sarcoidosis. Am J Clin Dermatol. 2014 Aug;15(4):283-97. doi: 10.1007/s40257-014-0079-3. PMID: 24820822.
- Karadağ AS, Parish LC. Sarcoidosis: A great imitator. Clin Dermatol. 2019 May-Jun;37(3):240-254. doi: 10.1016/j.clindermatol.2019.01.005. Epub 2019 Jan 15. PMID: 31178106.
- Caplan A, Rosenbach M, Imadojemu S. Cutaneous Sarcoidosis. Semin Respir Crit Care Med. 2020 Oct;41(5):689-699. doi: 10.1055/s-0040-1713130. Epub 2020 Jun 27. PMID: 32593176.
- Jain R, Yadav D, Puranik N, Guleria R, Jin JO. Sarcoidosis: Causes, Diagnosis, Clinical Features, and Treatments. J Clin Med. 2020 Apr 10;9(4):1081. doi: 10.3390/jcm9041081. PMID: 32290254; PMCID: PMC7230978.
- Ungprasert P, Ryu JH, Matteson EL. Clinical Manifestations, Diagnosis, and Treatment of Sarcoidosis. Mayo Clin Proc Innov Qual Outcomes. 2019 Aug 2;3(3):358-375. doi: 10.1016/j.mayocpiqo.2019.04.006. PMID: 31485575; PMCID: PMC6713839.
- Dai C, Shih S, Ansari A, Kwak Y, Sami N. Biologic Therapy in the Treatment of Cutaneous Sarcoidosis: A Literature Review. Am J Clin Dermatol. 2019 Jun;20(3):409-422. doi: 10.1007/s40257-019-00428-8. PMID: 30895525.
- Sand M, Sand D, Thrandorf C, Paech V, Altmeyer P, Bechara FG. Cutaneous lesions of the nose. Head Face Med. 2010 Jun 4;6:7. doi: 10.1186/1746-160X-6-7. PMID: 20525327; PMCID: PMC2903548.
- Chauhan R, Loewenstein SN, Hassanein AH. Rhinophyma: Prevalence, Severity, Impact and Management. Clin Cosmet Investig Dermatol. 2020 Aug 11;13:537-551. doi: 10.2147/CCID.S201290. PMID: 32848439; PMCID: PMC7429105.
- Hejazi EZ, Werth VP. Cutaneous Lupus Erythematosus: An Update on Pathogenesis, Diagnosis and Treatment. Am J Clin Dermatol. 2016 Apr;17(2):135-46. doi: 10.1007/s40257-016-0173-9. PMID: 26872954.
- Chauhan P, Adya KA. Dermatoscopy of Cutaneous Granulomatous Disorders. Indian Dermatol Online J. 2021 Jan 16;12(1):34-44. doi: 10.4103/idoj.IDOJ_543_20. PMID: 33768021; PMCID: PMC7982032.
- Muhjazi G, Gabrielli AF, Ruiz-Postigo JA, Atta H, Osman M, Bashour H, Al Tawil A, Husseiny H, Allahham R, Allan R. Cutaneous leishmaniasis in Syria: A review of available data during the war years: 2011-2018. PLoS Negl Trop Dis. 2019 Dec 12;13(12):e0007827. doi: 10.1371/journal.pntd.0007827. PMID: 31830034; PMCID: PMC6907761.
- Gurel MS, Tekin B, Uzun S. Cutaneous leishmaniasis: A great imitator. Clin Dermatol. 2020 Mar-Apr;38(2):140-151. doi: 10.1016/j.clindermatol.2019.10.008. Epub 2019 Oct 24. PMID: 32513395.
- Ul Bari A, Raza N. Lupoid cutaneous leishmaniasis: a report of 16 cases. Indian J Dermatol Venereol Leprol. 2010 Jan-Feb;76(1):85. doi: 10.4103/0378-6323.58698. PMID: 20061750.
- Chen Q, Chen W, Hao F. Cutaneous tuberculosis: A great imitator. Clin Dermatol. 2019 May-Jun;37(3):192-199. doi: 10.1016/j.clindermatol.2019.01.008. Epub 2019 Jan 11. PMID: 31178102.
- Bonamonte D, Filoni A, Verni P, Angelini G. Cutaneous Tuberculosis. In: Bonamonte D, Angelini G. (eds) Mycobacterial Skin Infections. Springer, Cham. 2017. https://doi.org/10.1007/978-3-319-48538-6_2
- Prasse A. The Diagnosis, Differential Diagnosis, and Treatment of Sarcoidosis. Dtsch Arztebl Int. 2016 Aug 22;113(33-34):565-74. doi: 10.3238/arztebl.2016.0565. PMID: 27598883; PMCID: PMC5015588.
- Spagnolo P, Rossi G, Trisolini R, Sverzellati N, Baughman RP, Wells AU. Pulmonary sarcoidosis. Lancet Respir Med. 2018 May;6(5):389-402. doi: 10.1016/S2213-2600(18)30064-X. Epub 2018 Apr 3. PMID: 29625772.
- Veltkamp M, Grutters JC. The Pulmonary Manifestations of Sarcoidosis. In: Judson M. (eds) Pulmonary Sarcoidosis. Respiratory Medicine, Humana Press, New York, NY. 2014; 17. https://doi.org/10.1007/978-1-4614-8927-6_2
- Bonifazi M, Gasparini S, Alfieri V, Renzoni EA. Pulmonary Sarcoidosis. Semin Respir Crit Care Med. 2017 Aug;38(4):437-449. doi: 10.1055/s-0037-1603766. Epub 2017 Jul 27. PMID: 28750459.
- Elavarasi A, Jain N, Rajeshwari M. Sarcoidosis: A rare cause of thyroiditis. Lung India. 2021 Sep-Oct;38(5):494-496. doi: 10.4103/lungindia.lungindia_121_21. PMID: 34472533; PMCID: PMC8509168.
- Fazzi P, Fallahi P, Ferrari SM. Sarcoidosis and Thyroid Autoimmunity. Front Endocrinol (Lausanne). 2017 Aug 8;8:177. doi: 10.3389/fendo.2017.00177. PMID: 28848497; PMCID: PMC5550685.
- Baughman RP, Valeyre D, Korsten P, Mathioudakis AG, Wuyts WA, Wells A, Rottoli P, Nunes H, Lower EE, Judson MA, Israel-Biet D, Grutters JC, Drent M, Culver DA, Bonella F, Antoniou K, Martone F, Quadder B, Spitzer G, Nagavci B, Tonia T, Rigau D, Ouellette DR. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021 Dec 16;58(6):2004079. doi: 10.1183/13993003.04079-2020. PMID: 34140301.
- Bangert CA, Costner MI. Methotrexate in dermatology. Dermatol Ther. 2007 Jul-Aug;20(4):216-28. doi: 10.1111/j.1529-8019.2007.00135.x. PMID: 17970887.
- Sakr MF. Tongue Fissures. In: Tongue Lesions. Springer, Cham. 2022. https://doi.org/10.1007/978-3-031-08198-9_13.