More Information
Submitted: April 22, 2022 | Approved: May 05, 2022 | Published: May 06, 2022
How to cite this article: Bereda G. Antimycolytic agents: fungistatic and fungicide. Ann Dermatol Res. 2022; 6: 001-009.
DOI: 10.29328/journal.adr.1001019
Copyright License: © 2022 Bereda G. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Antimycolytic; Fungistatic; Fungicide
Abbreviations: ADRS Adverse Drug Reactions; ATP: Adenine Triphosphate; CYP450: Cytochrome P450; DNA: Deoxyribonucleic; DI: Drug Interaction; 5FU: 5-Fluorouracil; PCM: Paracoccidioidomycosis; PH: Potenz Hydrogen; P-gp: Pglycoprotein; RNA: Ribonucleic Acid; SJS: Stevens Johnson Syndrome
Invasive fungal infections are described as a continuous and severe harm to human health and they are associated with at least 1.5 million deaths worldwide each year. Amphotericin B exerts its activity through hydrophobic interactions with cell membrane ergosterol, cause the rupturing or leakage of cell membrane. The antifungal azole medicine group is classified as imidazoles (clotrimazole, ketoconazole, miconazole) and triazoles (fluconazole, itraconazole, voriconazole, posaconazole, isavuconazole) that are named according to the number of nitrogen atoms in the azole ring. Flucytosine is a first-line treatment for the management of cryptococcal meningitis. The most routine adverse effects of fluconazole involve accelerated liver enzymes, gastrointestinal complaints, headache, and skin rash. If antacids, PPIs, H2 blockers administered together with ketoconazole medicines; they will reduce the blood levels of ketoconazole by increasing gastric pH because ketoconazole requires an acidic media for dissolution and systematic absorption. Griseofulvin ruptures mitotic spindle during metaphase by interacting with fungal microtubules-(-), fungal mitosis (metaphase arrest), adequate to block expansion of fungi (drug is static), preventing them from damaging.
Fungal infections are caused by microscopic organisms that can damage the epithelial tissue. The fungal kingdom involves yeasts, molds, rusts and mushrooms [1]. Fungi, like animals, are hetrotrophic, that is, they obtain nutrients from the ambient, not from endogenous origins (like plants with photosynthesis). Most fungi are advantageous and are included in biodegradation, nevertheless, a few can cause opportunistic infections if they are interpolated into the skin via wounds, or into the lungs and nasal route if inhaled [2]. Diseases caused by fungi include superficial infections of the skin by dermatophytes in the microsporum, trichophyton or epidermophyton genera. These dermophytic infections are named for the site of infection rather than the causative organism [3,4]. Invasive fungal infections are described as a continuous and severe harm to human health and they are associated with at least 1.5 million deaths worldwide each year [5,6]. Fungal infections are routine in immunocompromised patients, as displayed in their chemotherapy, acquired immune deficiency syndrome, and/or organ transplantation [7]. The infection caused by fungi of the genus pracoccidioides was first defined by Adolpho Lutz in 1908. In 1971, during the meeting of many mycologists from Latin America in Medellín-Colombia, the term Paracoccidioidomycosis was made to require the systemic granulomatous infection caused by the thermodimorphic fungi of the genus paracoccidioides [8,9].
Antimycolytic agents
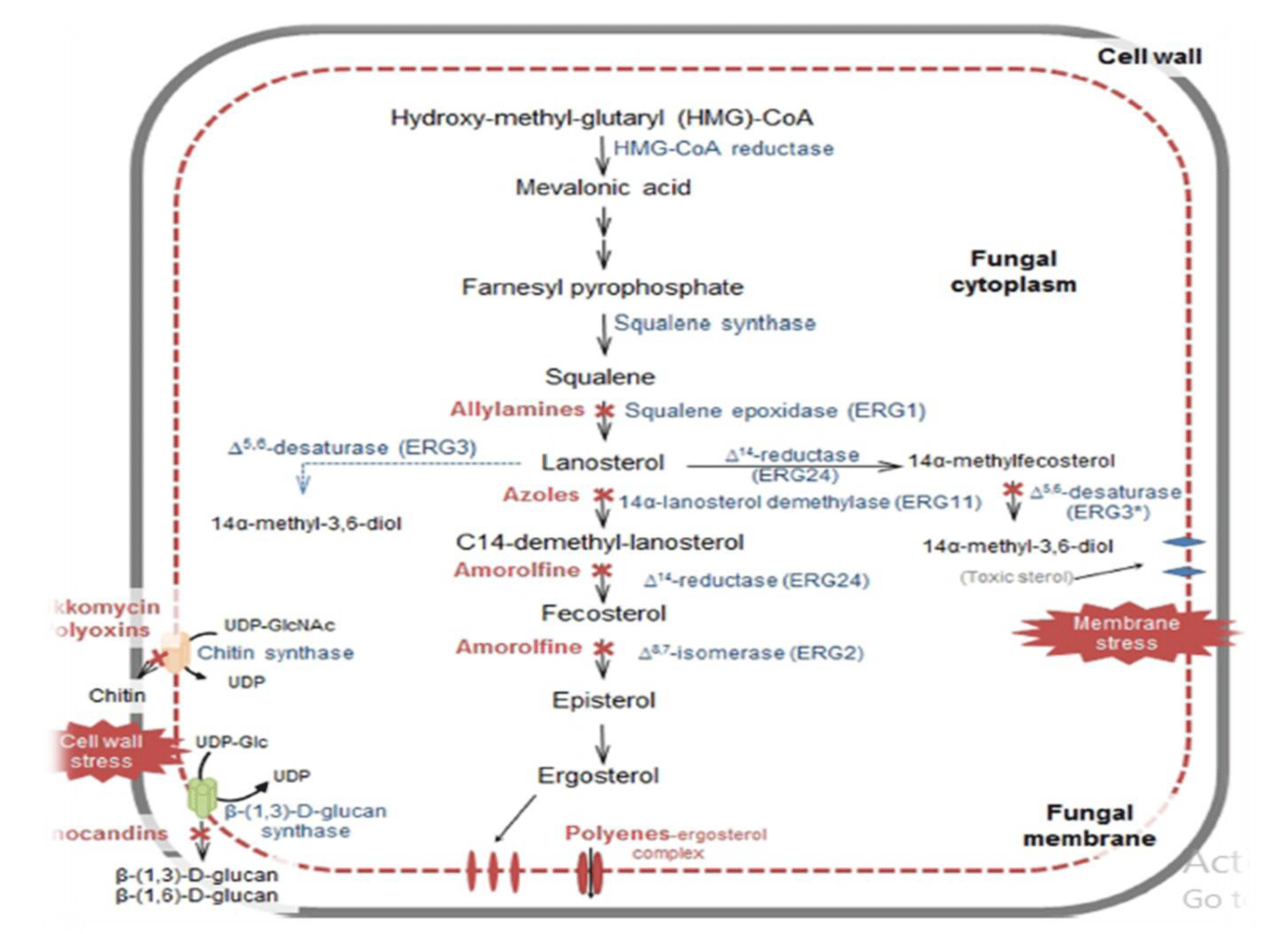
Recently, four antifungal classes of medication named as azoles (imidazoles such as ketoconazole, clotrimazole, miconazole, tioconazole, econazole and triazoles such as fluconazole, vorioconazole, itraconazole, posaconazole), polyenes (amphotericin B and nystatin), pyrimidines (flucytosine) and echinocandins (caspofungin, micafungin and anidulafungin) are mostly used by clinicians and veterinarians for systemic treatment. Antifungals have various drawbacks in terms of toxicity, spectrum of activity, safety and pharmacokinetic properties [10,11] (Figure 1).
Figure 1: Schematic illustration of antifungal agents’ site of actions.
Antifungal antibiotics (polyenes): i. Amphotericin B; ii. Nystatin.
Amphotericin B
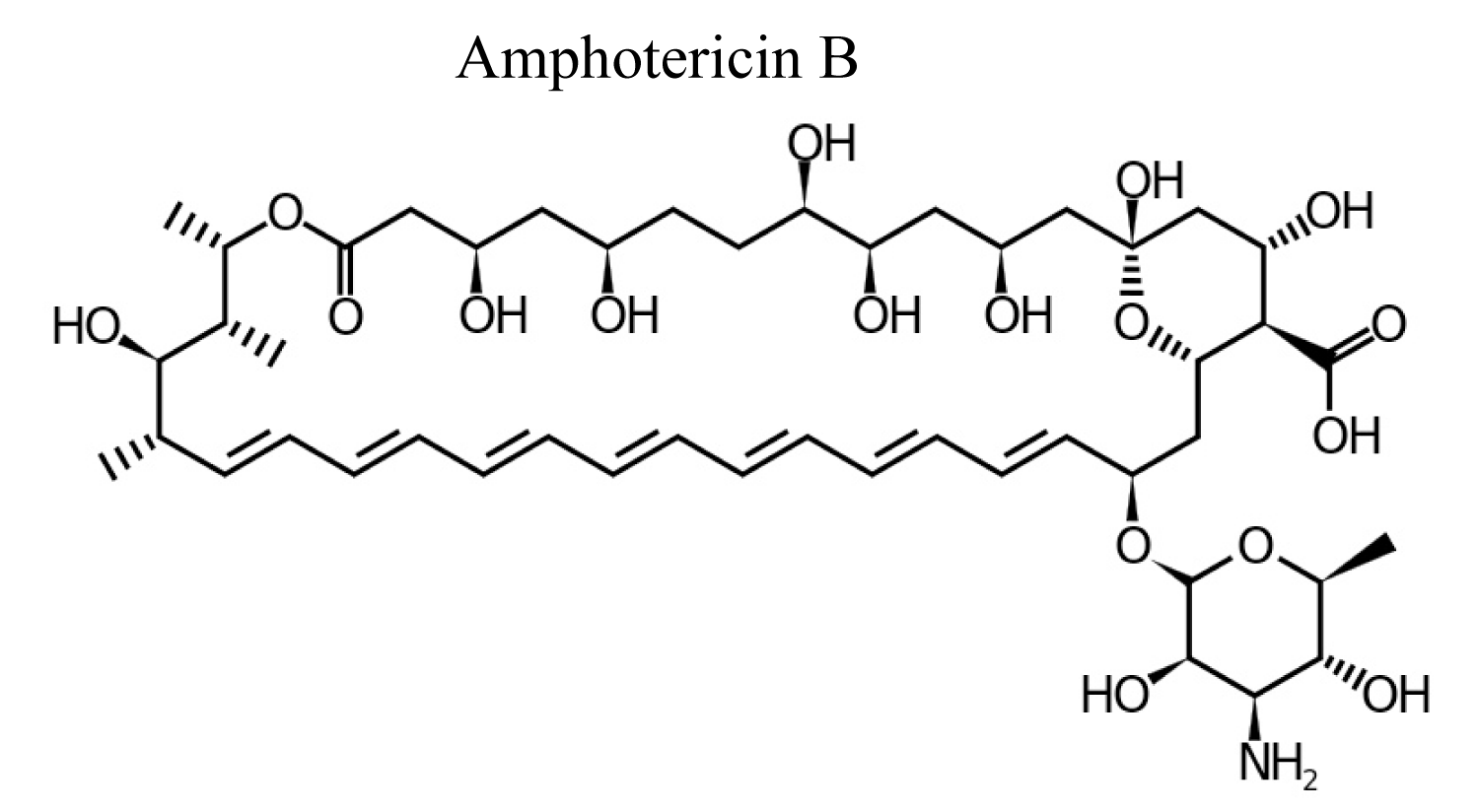
Amphotericin-B is polyene antibiotic and it is produced by bacterial strain of streptomyces nodusus using fermentation with antifungal activity. Amphotericin-B is an amphipathic polyene antibiotic which permeabilizes ergosterol-containing membranes [12] (Figure 2).
Figure 2: Chemical structure of Amphotericin B.
Mechanism of action: Amphotericin-B binds to the sterols in fungal cell membrane and generates transmembrane channel and electrolyte leakage. Amphotericin B exerts its activity through hydrophobic interactions with cell membrane ergosterol, cause the rupturing or leakage of cell membrane and pores formation permits the efflux of potassium, eventually leading to cell death [13].
Spectrum of activity: Amphotericin B has high activity against cryptococcus spp and most candida spp, candida albicans, histoplasma capsulatum, blastomyces dermatitidis, coccidioides immitis, aspergillus, rhodotorula with the exception of candida lusitaniae, which commonly is found to have greater minimal inhibitory concentrations [14,15].
Clinical use: Amphotericin B was the first antifungal medicine developed and is confirmed for the treatment of multiple invasive fungal infections involving candidiasis, aspergillosis, cryptococcosis, blastomycosis, histoplasmosis, mucormycosis, and sporotrichosis. Amphotericin B injection is used for treatment of severe and potentially life-threatening fungal infections [16,17].
Adverse drug effects: Intravenous amphotericin is toxic, causing fever, chills, hypotension during infusion, nephrotoxicity, electrolyte abnormalities and transient bone marrow suppression. Kidney damage is the most severe adverse effect of amphotericin B. Systemic toxicity (particularly nephrotoxicity) of amphotericin is lowered by using the liposomal/ lipid/micellar formulations. Certain side effects of Amphotericin B are anaphylactic reaction, leukoencephalopathy, cardiac arrhythmias, convulsions, and cardiorespiratory collapse [18].
Contraindications: Amphotericin B is not given for patients who have documented history of hypersensitivity; for nursing mother and individuals who take anticancer medications [19].
Drug interaction: If amphotericin-B is combined with 5-flucytosine; they have synergistic effect and used in severe infections and immunosuppressed patients [20,21]. Concomitant administration of vancomycin and aminoglycoside (both increase risk of nephrotoxicity) with amphotericin-B increases the risk of renal impairment and electrolyte disturbances. Coadministration of amphotericin B with immunosuppressants, such as tacrolimus or cyclosporine, in transplant recipients may be increases the risk of bone marrow suppression or amphotericin B accelerates myelosuppressive toxicity of antineoplastic medication. If ketoconazole and amphotericin-B are administered concurrently they have antagonistic/contraindicated effects [22,23].
Antimetabolite/fluorinated pyrimidine: Flucytosine
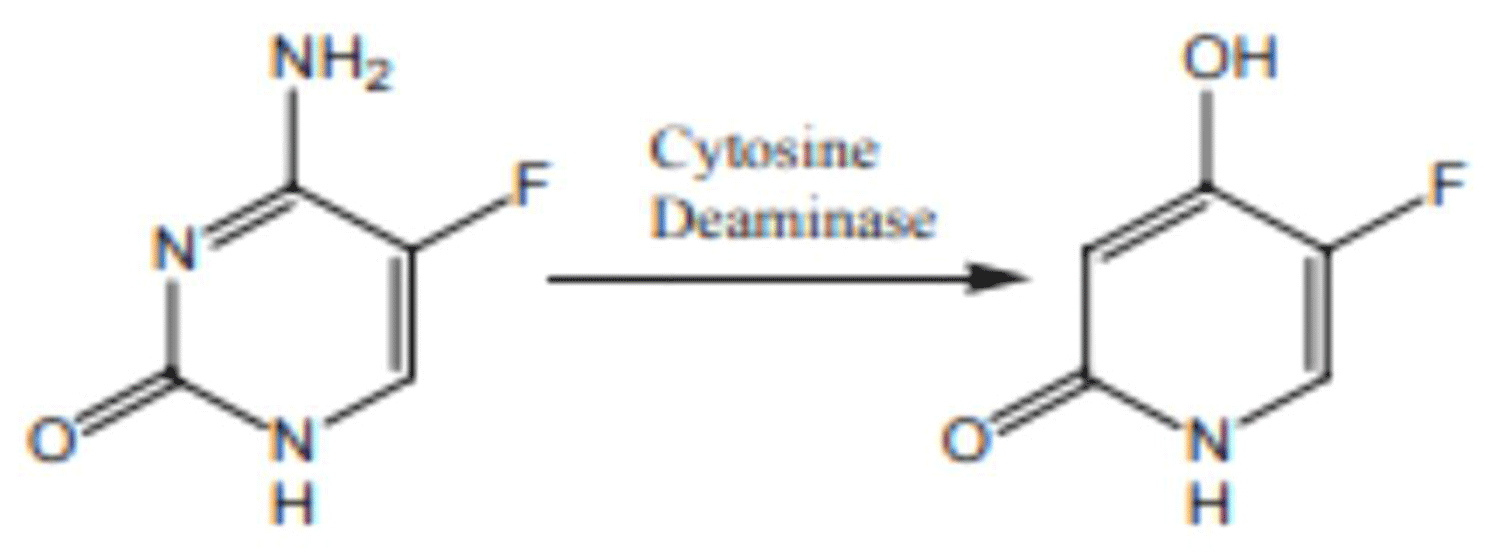
Flucytosine is antimetabolite that enters fungal cells via cytosine permease where it is converted to fluorouracil, which acts as false nucleoside inhibiting synthesis of both DNA and RNA [24] (Figure 3).
Figure 3: Chemical structure of Flucytosine.
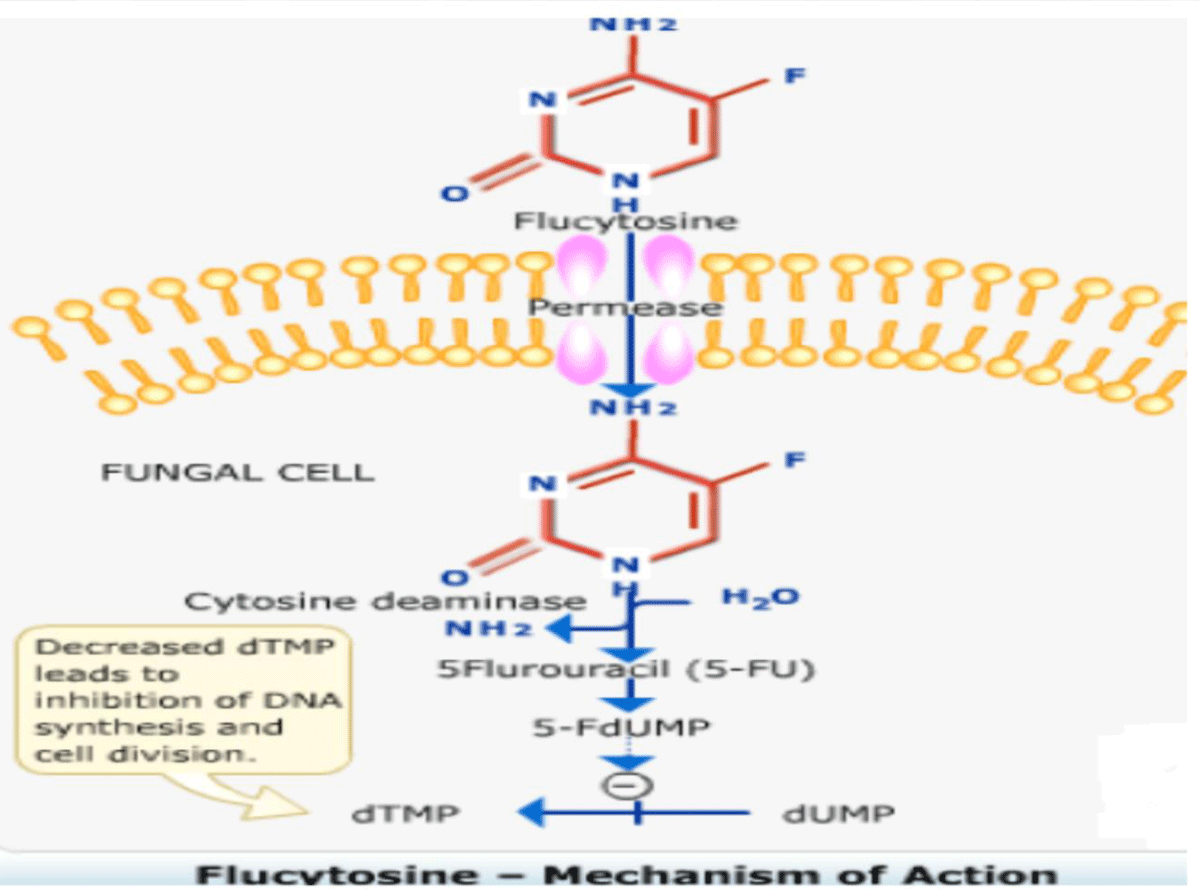
Mechanism of action: 5-Fluorouracil impairs synthesis of nucleic acid, eventually interfering with protein synthesis as well and also blocks thymidylate synthetase and thus DNA synthesis mammalian cells remain unaffected except few bone marrow cells. The drug enters the fungal cell via active transport on ATPases that normally transport pyrimidines. Once inside cells, fungal cytosine deaminase change the medicine to active 5FU, a very effective antitumor agent, which is integrated into RNA causing faulty RNA synthesis and also is a tough, non-competitive inhibitor of thymidylate synthesis interrupting the one carbon pool substrate. Mammalian cells do not contain cytosine deaminase [24-29] (Figure 4).
Figure 4: Mechanism of action of Flucytosine.
Spectrum of activity: Flucytosine is act on routine pathogenic treatment. Its spectrum of activity involves multiple candida spp, involving C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis. C krusei and C. lusitaniae are also involved in the spectrum but MICs are greater [30,31].
Clinical use: Flucytosine is a first-line treatment for management of cryptococcal meningitis and administered with amphotericin B during the induction period. Whereas, flucytosine has high activity against most candida spp, resistance elaborates hastily during use, limiting its treatment potential as a single agent [32,33].
Adverse drug effects: Flucytosine cause mild BM depression; loss of hair; and dose should be decreased in the presence of renal impairment [34].
Contraindications: Flucytosine is not indicated for individuals who are hypersensitive to the drugs and its derivatives [35].
Drug interaction: Concomitant administration of flucytosine with NSAIDs such as naproxen or ibuprofen may be increases the risk of kidney problems. Concurrent administration of flucytosine with cotrimoxazole, cancer chemotherapy and etc they aggravate bone marrow suppression [36,37].
Azoles (imidazoles and triazoles)
The azole antifungal drug is classified as imidazoles (clotrimazole, ketoconazole, miconazole) and triazoles (fluconazole, itraconazole, voriconazole, posaconazole, isavuconazole) that are named corresponding to the number of nitrogen atoms in the azole ring [3,38].
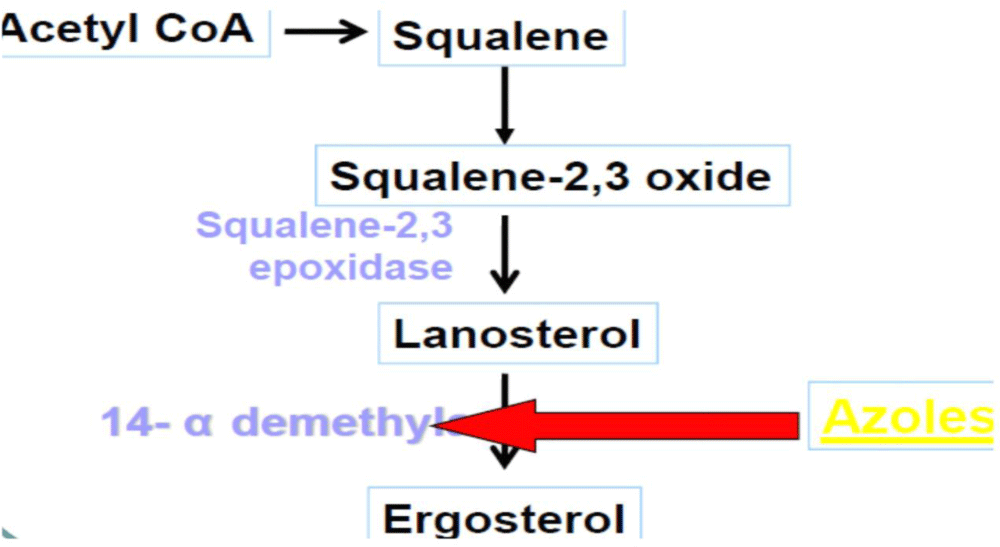
Mechanism of action: Azoles exert their action by inhibiting the fungal cytochrome P450 3A enzyme (lanosine 14α-demethylase) demethylation of lanosterol in fungi, which interferes with the synthesis of ergosterol in the fungal cell membrane [41].
Ketoconazole
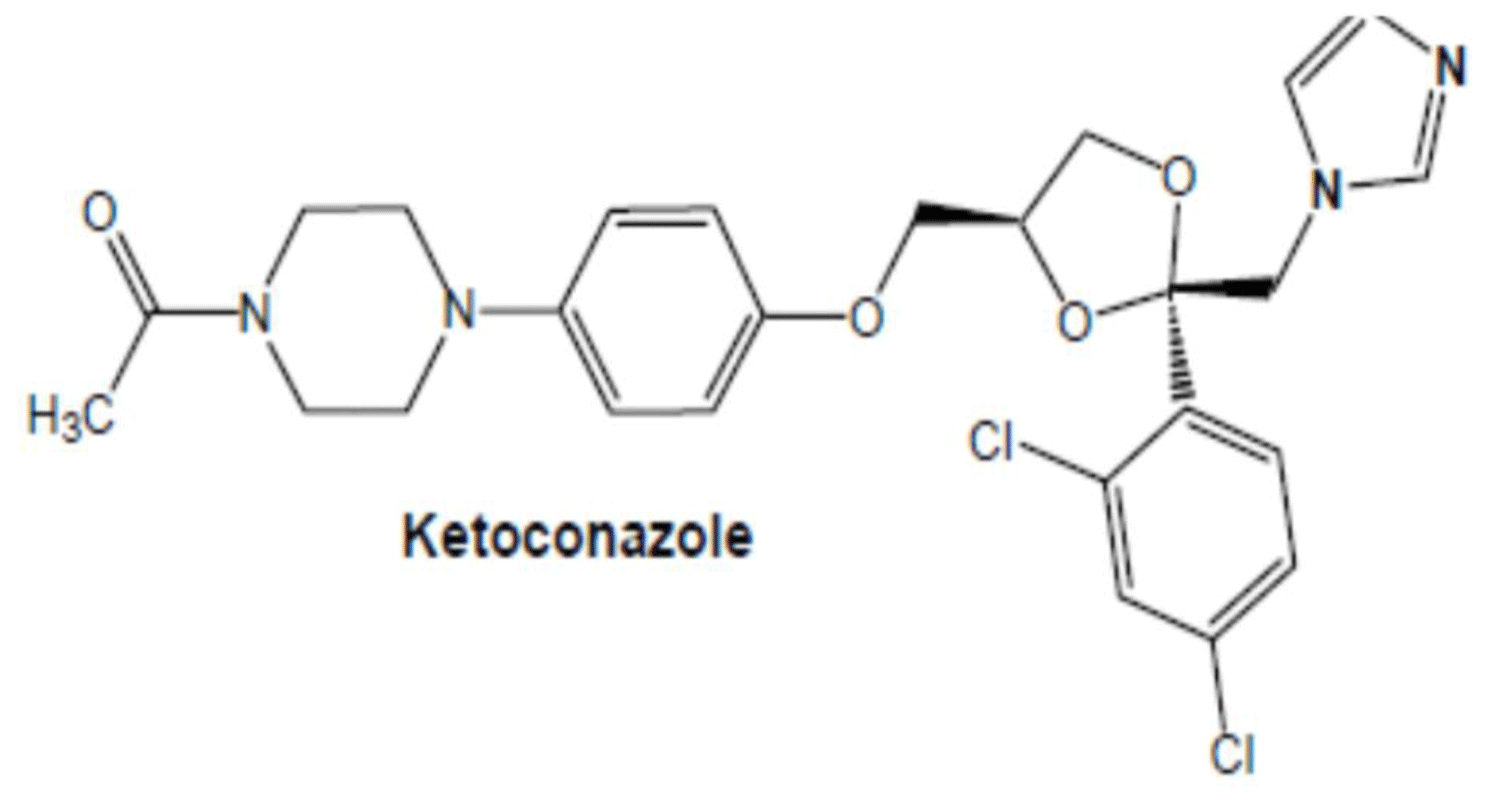
Ketoconazole is a broad spectrum antifungal agent that belongs to biopharmaceuticals classification system (BCS) class II drug means ketoconazole has low solubility and high permeability which exhibits dissolution rate-limited absorption [40] (Figure 5).
Figure 5: Chemical structure of Ketoconazole.
Mechanism of action: Ketoconazole is act by inhibiting the synthesis of ergosterol, the fungal equivalent of cholesterol, thereby elevating membrane fluidity and suppressing growth of the fungus [41] (Figure 6).
Figure 6: Mechanism of action of Ketoconazole.
Spectrum of activity: Ketoconazole is rarely used at present for treatment or prophylaxis of fungal infections. Ketoconazole is used for treatment of infections caused by a fungus and yeast [42,43].
Adverse drug reactions: Ketoconazole cause nausea and vomiting, worse with greater doses (800 mg/day) and also cause hepatoxicity, accelerate in transaminases, hepatitis, anti-androgenic effects decreasing of CYP P450 responsible for testosterone synthesis (dose related). Gynecomastia, oligosperma, decreased libido dose-related blockage of CYP P450 responsible for adrenal cortisol synthesis is ketoconazole mild side effects [44,45].
Contraindications: Ketoconazole is not indicated for individuals who have previously liver disease; porphyria; hypersensitivity to the drugs and its derivatives [46].
Drug interaction: If antacids, PPIs, H2 blockers administered together with ketoconazole medicines; they will reduce the blood levels of ketoconazole by increasing gastric pH because ketoconazole requires an acidic media for dissolution and systematic absorption. If ketoconazole administered with potent inhibitor of cytochrome P450 3A4 such as rifampin and phenytoin; they decrease ketoconazole blood levels. If ketoconazole administered with cyclosporin, warfarin, and astemizole, corticosteroid, and theophylline; it increases their blood levels. If ketoconazole is given together with an inhibitor of multiple CYP isozymes and P-gp; it can importantly accelerate serum concentrations of multiple medicines [47-49].
Fluconazole
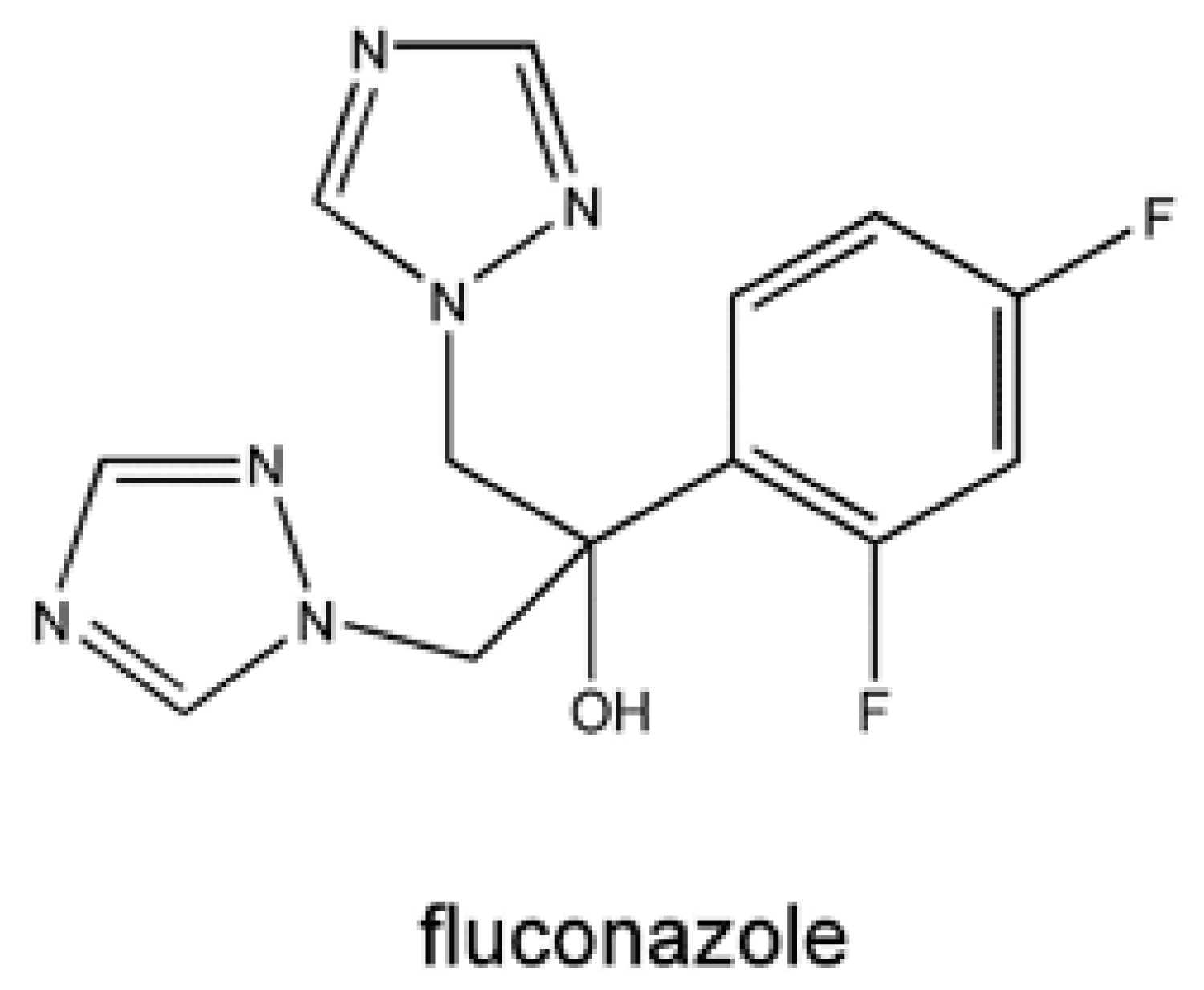
The pharmacokinetic characteristics of the individual azole medicines are different due to their variation in molecular weight, solubility, and protein binding. Fluconazole is idiomatic due to its low molecular weight and great aqueous solubility. It establishes great bioavailability, approximately 90%, and its absorption is not affected by gastric acidity or food [50-53] (Figure 7).
Figure 7: Chemical structure of Fluconazole.
Spectrum of activity: Fluconazole is active against multiple medically significant candida spp, involving C. albicans, C. parapsilosis, C. tropicalis, C. lusitaniae, and C. dubliniensis. It has reasonable activity against coccidioides spp. and cryptococcus neoformans and certain activity against Histoplasma capsulatum. The medication has no clinically important activity against most molds, involving aspergillus spp., fusarium spp., and the mucorales (previously called Zygomycetes), such as mucor spp. and rhizopus spp [54-56].
Clinical use: Fluconazole is indicated for treatment of both mucosal and systemic candidiasis, the treatment of cryptococcosis, and prophylaxis for candidiasis [57].
Adverse drug reactions: The most routine adverse effects of fluconazole involve accelerated liver enzymes, gastrointestinal complaints, hepatotoxicity, headache, and skin rash involving SJS. Fluconazole is used cautiously in patients with renal impairment [58,59].
Contraindications: Fluconazole is not given for individuals who are hypersensitive to the medicine and its derivatives [60].
Drug interactions: Fluconazole administration perhaps accelerates the consequences of oral hypoglycemic and decreases the metabolism of phenytoin and warfarin. Concomitant use of fluconazole with other medicines known to extend the QT interval, especially those metabolized by CYP2C9, 2C19 or 3A4, perhaps increase the risk of QT prolongation and torsades de pointes. Taking fluconazole with the potent CYP enzyme inducer such as warfarin, theophylline, rifampin etc can decrease fluconazole serum concentrations, possibly to subtherapeutic levels [61,62].
Itraconazole
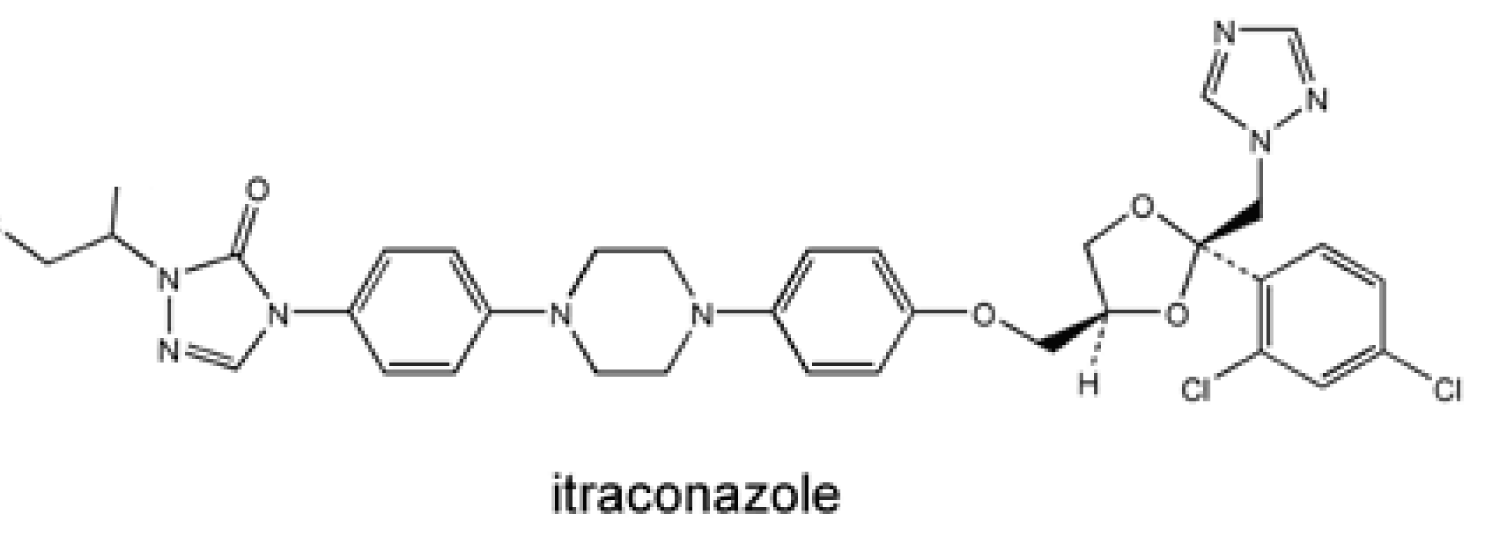
Absorption of the itraconazole capsule formulation is comparatively 55% but it is improved with gastric acidity and food intake. Therefore, it is recommended to be administered with an acidic beverage and food [63-65] (Figure 8).
Figure 8: Chemical structure of Itraconazole.
Spectrum of activity: Like fluconazole, itraconazole substantiates activity against most candida spp, with greater MICs for C. glabrata and C. krusei/Itraconazole has a wider spectrum of activity than fluconazole and also active against a broad spectrum’ of fungi involving C. neoformans, aspergillus spp., alastomyces dermatitidis, coccidioides spp., h. capsulatum, paracoccidioides brasiliensis, sporothrix spp., and dermatophytes. It is also active against most species of candida. Itraconazole has no clinically important activity against fusarium spp. or the mucorales and has variable activity against scedosporium spp [66,67].
Clinical use: Itraconazole is confirmed for the treatment of many mycoses, involving blastomycosis, mucosal candidiasis, coccidioidomycosis, cryptococcosis, histoplasmosis, onycho-mycosis, and sporotrichosis [68-70].
Adverse drug reactions: The most routine adverse effects of oral itraconazole are nausea, diarrhea, vomiting, pulmonary oedema, menstrual disorders, heart impairment, dyspepsia, abdominal pain, hypokalemia, elevated liver enzyme values, anaphylaxis, liver injury, and rash involving SJS and severe hepatic toxicity [71,72].
Drug interactions: Absorption of the itraconazole oral capsules and the posaconazole oral solution is optimized by gastric acidity, so proton pump inhibitors and histamine-2 blockers should be avoided because they minimize gastric acidity of the stomach which is required for the absorption of itraconazole oral capsules and the posaconazole oral solution derivatives. Taking itraconazole with a CYP3A4 substrate that extends the QT interval increases the risk of QT prolongation and torsades de pointes. Itraconazole is also a P-gp inhibitor and can accelerate serum concentrations of P-gp substrates [73-75].
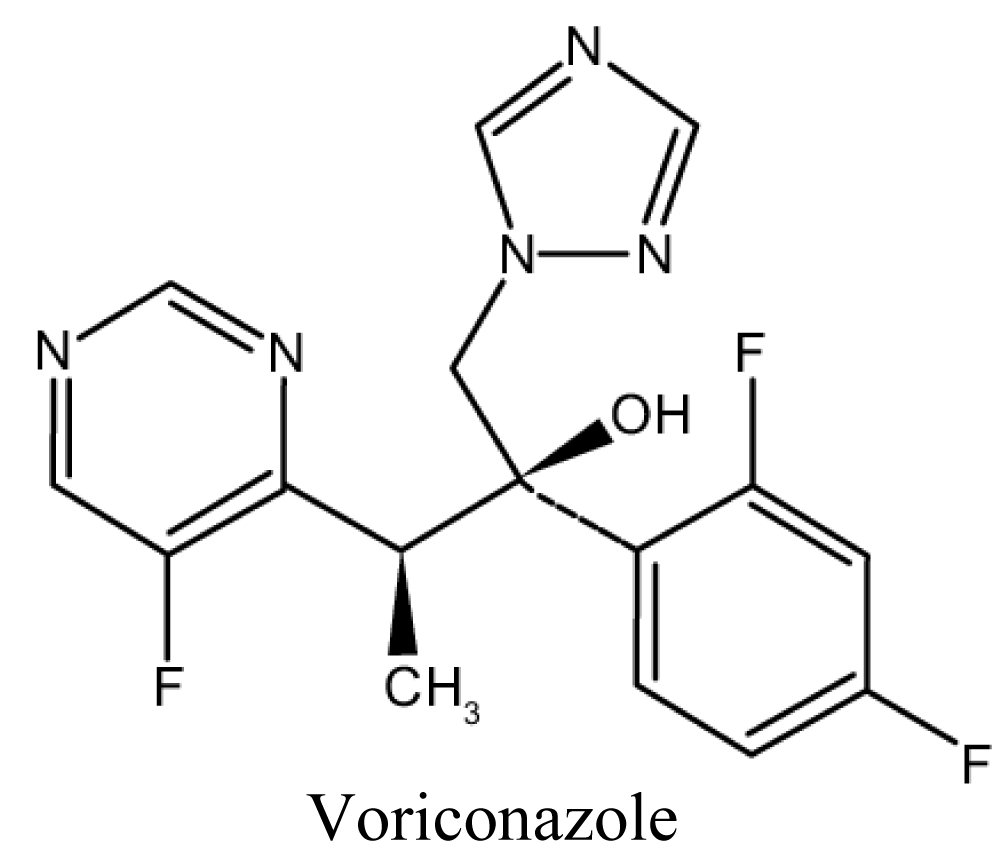
Voriconazole
Absorption is not affected by gastric acidity and is optimal in the fasted state. Loading doses for the first 24 hours are recommended to more rapidly achieve therapeutic levels [76,77] (Figure 9).
Figure 9: Chemical structure of Voriconazole.
Spectrum of activity: Voriconazole has a spectrum of activity identical to that of itraconazole, but is clinically measured to be more active against aspergillus spp. and most species of candida, involving C. glabrata and C. krusei. Voriconazole is active against fusarium spp. and scedosporium spp, but it is not active against the mucorales; infection with these organisms has advanced during treatment with voriconazole [78,79].
Clinical use: Voriconazole is confirmed for the treatment of invasive aspergillosis, esophageal candidiasis, invasive candidiasis, scedosporiosis, and fusariosis [80].
Adverse drug reactions: In general, the triazole medicines are fairly well-tolerated. Side-effects enclose rash, headache, or gastrointestinal upset. Hepatotoxicity marked by acceleration of liver chemistry tests and, least routinely, liver failure, is the most routine and severe group of side effect. Long-term administration of voriconazole perhaps cause painful periostitis of long bones, premature aging, and an elevated occurrence of squamous cell carcinoma or melanoma in sun-exposed skin transient visual disturbances involving blurred vision, photophobia and changed perception of color or image have happened in about 20% of patients treated with voriconazole [81].
Contraindications: Voriconazole is not indicated for breasting mother; for individuals who are hypersensitive to drugs and its derivatives [82].
Drug interactions: The triazoles can cause QT prolongation due to drug–drug interactions perhaps happened by the additive effect of additional QT prolonging agents [83,84]. Voriconazole is a substrate of CYP2C19, 2C9 and 3A4. Medicines that block or induces alone or most of these pathways perhaps importantly alter serum concentrations of voriconazole. Concomitant use of voriconazole with other medicines that extend the QT interval, particularly those metabolized by CYP2C9, 2C19 or 3A4, perhaps increase the risk of QT prolongation and torsades de pointes [85].
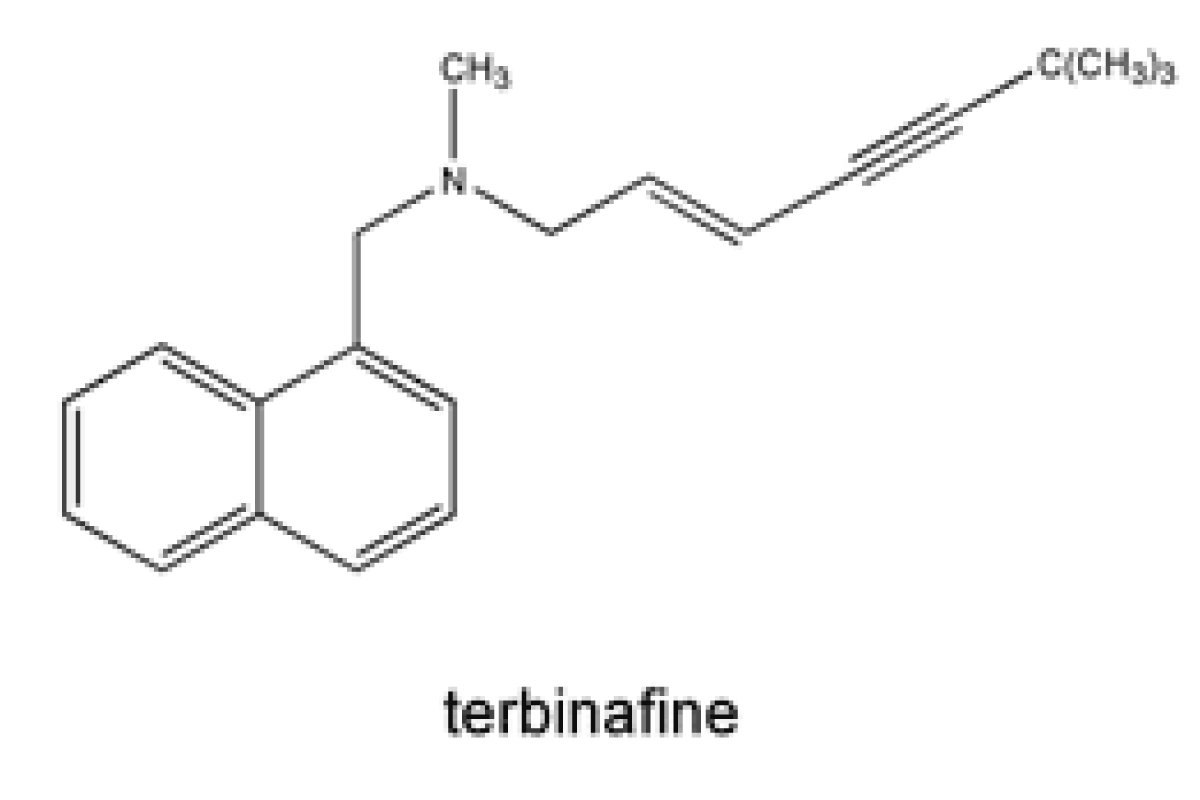
Allylamine e.g. Terbinafine
Terbinafine is a synthetic allylamine antifungal. Terbinafine is a highly lipophilic base and consequently has very high volume of distribution a strong and non-specific binding to plasma proteins [86] (Figure 10).
Figure 10: Chemical structure of Terbinafine.
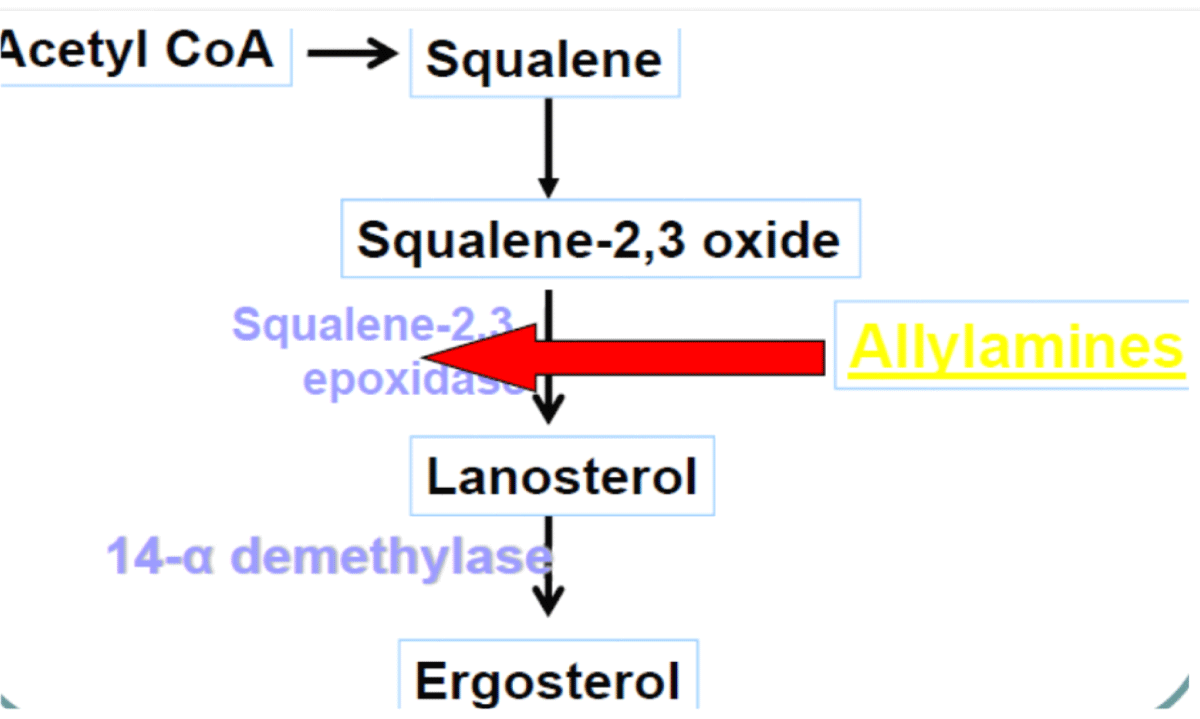
Mechanism of action: Terbinafine acts by interfering with ergosterol biosynthesis by blocks the fungal enzyme squalene epoxidase. This leads to the concentration of the sterol squalene, which is toxic to the organism [87] (Figure 11).
Figure 11: Mechanism of action of Terbinafine.
Use: Terbinafine is used for treatment of superficial mycoses (fingernail, toenail); systematic mycoses [88].
Adverse drug reaction: Orally administered terbinafine may be cause nausea, vomiting, changed taste, headache, and tiredness. Topical application of terbinafine perhaps causes burning, stinging, redness, itchiness, and drying of the skin [89].
Contraindications: Terbinafine is not given for breasting mother; individuals who have active or severe liver disease; those who are hypersensitive to the medications and its derivatives.
Drug interaction: Coincident administration of fluconazole and terbinafine perhaps accelerates the serum concentrations of terbinafine. Cimetidine perhaps decreases the clearance of terbinafine if administered together, and enzyme inducers drugs such as rifampin perhaps increase terbinafine clearance if administered concomitantly [90].
Other antibiotics (Griseofulvin)
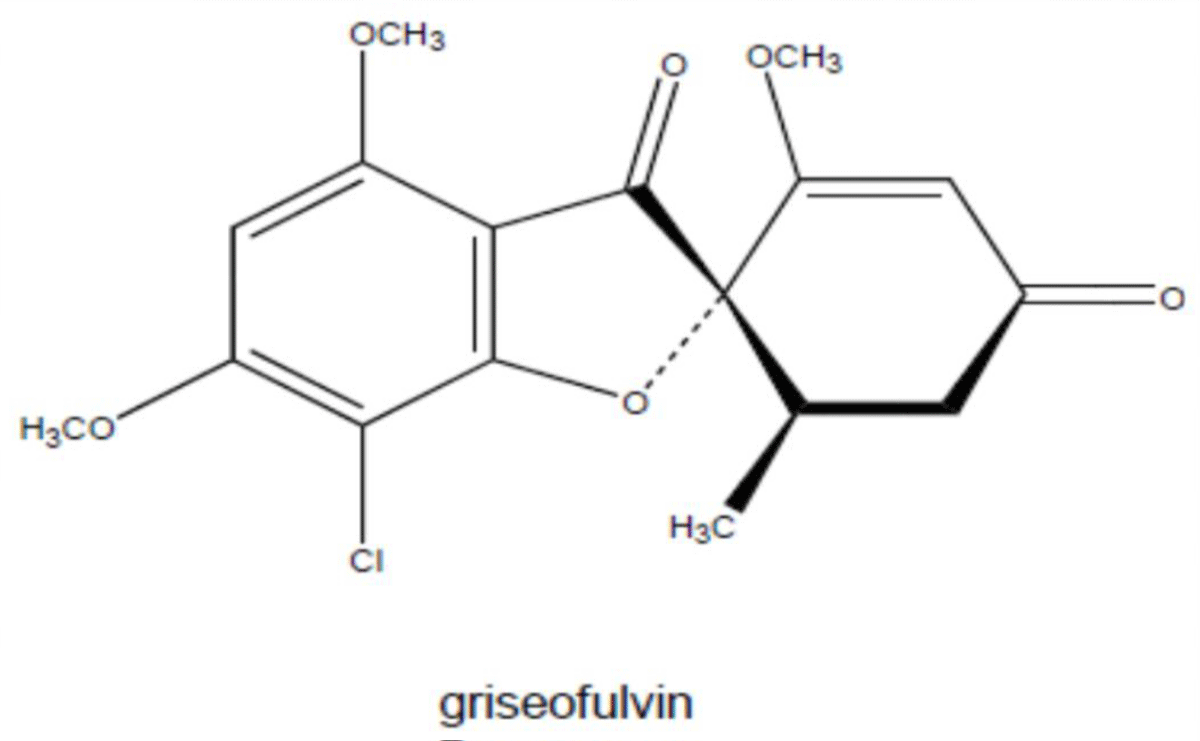
Griseofulvin is antibiotic produced by the mycelial fungus penicillium patulum. It is a mycotoxic metabolic product of penicillium spp [91] (Figure 12).
Figure 12: Chemical structure of Griseofulvin.
Mechanism of action: Griseofulvin ruptures the mitotic spindle during metaphase by interacting with fungal microtubules-(-), fungal mitosis (metaphase arrest), which is adequate to block growth of fungi (medicine is static), eventually damaging fungi cell membrane [91].
Spectrum of activity: Griseofulvin are used for the treatment of systemic antifungal to manage topical ringworm infections, e.g., onychomycosis, tinea capitis, tinea pedis, etc [92].
Use: Griseofulvin is indicated for treament tinea capitis; tinea unguium; pedis caused by fungi and griseofulvin used for treatment of skin infections such as athletes foot, jock itch etc.
Adverse drug reactions: Griseofulvin cause GI disturbances, allergic reactions, skin rash, headache, photosensitivity, angioedema, peripheral neuritis, lethargy, mental confusion, blurring of vision, vertigo, being an antimiototic (bone marrow suppression), leucopenia, neutopenia [93].
Contraindications: Griseofulvin is not given for patients who have porphyria; severe liver disease.
Drug interactions: If griseofulvin administered with OADs and OCs, it antagonizes their effects. Concurrent administration of griseofulvin with AEDs such as phenobarbital, is decreases the gastrointestinal absorption of AEDs. Concomitant administration of griseofulvin with CYP450 enzyme inducers medications such as hypnotic’s perhaps decreases plasma concentrations of griseofulvin. If griseofulvin given with alcohol, it causes disulfiram like reaction [94].
Fungi, like animals, are hetrotrophic, that is, they obtain nutrients from the ambient, not from endogenous origins (like plants with photosynthesis). Amphotericin B was the first antifungal drug developed and is confirmed for the treatment of multiple invasive fungal infections enclosing candidiasis, aspergillosis, cryptococcosis, blastomycosis, histoplasmosis, mucormycosis, and sporotrichosis. Intravenous amphotericin is toxic, causing fever, chills, hypotension during infusion, nephrotoxicity, electrolyte abnormalities and transient bone marrow suppression. The absorption of itraconazole is decreased by medicines that accelerate gastric pH, such as antacids, H2-receptor blockers and proton pump inhibitors. Orally administered terbinafine perhaps cause nausea, vomiting, altered taste, headache, and tiredness. Topical application of terbinafine perhaps causes burning, stinging, redness, itchiness, and drying of the skin.
The author would be grateful to anonymous reviewers for the comments that increase the quality of this manuscript.
Data sources: Sources searched include Google Scholar, Research Gate, PubMed, NCBI, NDSS, PMID, PMCID, and Cochrane database. Search terms included: antimycolytic agents both fungistatic and fungicide.
- Amara AA. Improving Animal Immunity to Prevent Fungal Infections with Folk Remedies and Advanced Medicine. In Fungal Diseases in Animals. 2021;127-162.
- Watkinson SC. Physiology and adaptation. In The Fungi 2016 Jan 1;141-187.
- Dutt K. Role of Antifungal Drugs in Combating Invasive Fungal Diseases. High Value Fermentation Products: Human Health. 2019 Mar 12;1:103-44.
- Hubka V, Peano A, Cmokova A, Guillot J. Common and emerging dermatophytoses in animals: well-known and new threats. InEmerging and Epizootic Fungal Infections in Animals 2018;31-79.
- Pianalto KM, Alspaugh JA. New Horizons in Antifungal Therapy. J Fungi (Basel). 2016 Oct 2;2(4):26. doi: 10.3390/jof2040026. PMID: 29376943; PMCID: PMC5715934.
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012 Dec 19;4(165):165rv13. doi: 10.1126/scitranslmed.3004404. PMID: 23253612.
- Pianalto KM, Alspaugh JA. New Horizons in Antifungal Therapy. J Fungi (Basel). 2016 Oct 2;2(4):26. doi: 10.3390/jof2040026. PMID: 29376943; PMCID: PMC5715934.
- Carmona EM, Limper AH. Overview of Treatment Approaches for Fungal Infections. Clin Chest Med. 2017 Sep;38(3):393-402. doi: 10.1016/j.ccm.2017.04.003. Epub 2017 May 31. PMID: 28797484.
- Shikanai-Yasuda MA. PARACOCCIDIOIDOMYCOSIS TREATMENT. Rev Inst Med Trop Sao Paulo. 2015 Sep;57 Suppl 19(Suppl 19):31-7. doi: 10.1590/S0036-46652015000700007. PMID: 26465367; PMCID: PMC4711189.
- Mendes RP, Cavalcante RS, Marques SA, Marques MEA, Venturini J, Sylvestre TF, Paniago AMM, Pereira AC, da Silva JF, Fabro AT, Bosco SMG, Bagagli E, Hahn RC, Levorato AD. Paracoccidioidomycosis: Current Perspectives from Brazil. Open Microbiol J. 2017 Oct 31;11:224-282. doi: 10.2174/1874285801711010224. PMID: 29204222; PMCID: PMC5695158.
- Mendes RP, Cavalcante RS, Marques SA, Marques MEA, Venturini J, Sylvestre TF, Paniago AMM, Pereira AC, da Silva JF, Fabro AT, Bosco SMG, Bagagli E, Hahn RC, Levorato AD. Paracoccidioidomycosis: Current Perspectives from Brazil. Open Microbiol J. 2017 Oct 31;11:224-282. doi: 10.2174/1874285801711010224. PMID: 29204222; PMCID: PMC5695158.
- Lenz KD, Klosterman KE, Mukundan H, Kubicek-Sutherland JZ. Macrolides: From Toxins to Therapeutics. Toxins (Basel). 2021 May 12;13(5):347. doi: 10.3390/toxins13050347. PMID: 34065929; PMCID: PMC8150546.
- Nasr G, Greige-Gerges H, Elaissari A, Khreich N. Liposomal membrane permeability assessment by fluorescence techniques: Main permeabilizing agents, applications and challenges. Int J Pharm. 2020 Apr 30;580:119198. doi: 10.1016/j.ijpharm.2020.119198. Epub 2020 Mar 10. PMID: 32169353.
- Drew RH. Polyenes for prevention and treatment of invasive fungal infections. InAntifungal therapy 2019 Mar 8; 155-175.
- Drew RH, Townsend ML, Pound MW, Johnson SW. Antifungal use in transplant recipients: Selection, administration, and monitoring. InAntifungal Therapy 2019 Mar 8;403-443.
- McKeny PT, Nessel TA, Zito PM. Antifungal antibiotics. StatPearls [Internet]. 2020 Sep 29.
- Kaushik A, Kest H. The Role of Antifungals in Pediatric Critical Care Invasive Fungal Infections. Crit Care Res Pract. 2018 Nov 22;2018:8469585. doi: 10.1155/2018/8469585. PMID: 30595916; PMCID: PMC6282141.
- Mourad A, Perfect JR. Tolerability profile of the current antifungal armoury. J Antimicrob Chemother. 2018 Jan 1;73(suppl_1):i26-i32. doi: 10.1093/jac/dkx446. PMID: 29304209; PMCID: PMC6636388.
- Karimzadeh I, Khalili H, Sagheb MM. Preventing or attenuating amphotericin B nephrotoxicity with dopamine receptor agonists: a literature review. Trends in Pharmaceutical Sciences. 2015 Sep 1;1(3):129-38.
- Bellmann R, Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. 2017 Dec;45(6):737-779. doi: 10.1007/s15010-017-1042-z. Epub 2017 Jul 12. PMID: 28702763; PMCID: PMC5696449.
- Bustamante B, Hidalgo JA, Campos PE. Antifungal Drugs. InCurrent Progress in Medical Mycology 2017;29-89.
- Carreno JJ, Kenney RM, Lomaestro B. Vancomycin-associated renal dysfunction: where are we now? Pharmacotherapy. 2014 Dec;34(12):1259-68. doi: 10.1002/phar.1488. Epub 2014 Sep 15. PMID: 25220436.
- Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012 Sep;68(9):1243-55. doi: 10.1007/s00228-012-1259-9. Epub 2012 Mar 13. PMID: 22411630.
- Maloy JP. Characterization of an Intracellular Flagellar System in Pathogenic Burkholderia Species. University of California, Los Angeles; 2017.
- Abdelgadir HE. Eumycetoma and Plant-based Pharmacotherapy Candidates (Doctoral dissertation, University of Gezira).
- Odiba AS. Molecular profiling and phylogenetic analysis of the erg11 gene of fluconazole resistant strains of candida species isolated from humans and dogs of reproductive age (Doctoral dissertation, University of Nigeria, Nsukka).
- Ward NP, DeNicola GM. Sulfur metabolism and its contribution to malignancy. Int Rev Cell Mol Biol. 2019;347:39-103. doi: 10.1016/bs.ircmb.2019.05.001. Epub 2019 Jun 17. PMID: 31451216.
- Lewis RE, Fothergill AW. Antifungal agents. InDiagnosis and Treatment of Fungal Infections 2015;79-97.
- Carlsson M. Metabolic regulation and anticancer drug resistance in the yeast Saccharomyces cerevisiae. 2014 Aug 19.
- Makanjuola O, Bongomin F, Fayemiwo SA. An Update on the Roles of Non-albicansCandida Species in Vulvovaginitis. J Fungi (Basel). 2018 Oct 31;4(4):121. doi: 10.3390/jof4040121. PMID: 30384449; PMCID: PMC6309050.
- Yousfi H, Cassagne C, Ranque S, Rolain JM, Bittar F. Repurposing of Ribavirin as an Adjunct Therapy against Invasive Candida Strains in an In Vitro Study. Antimicrob Agents Chemother. 2019 Sep 23;63(10):e00263-19. doi: 10.1128/AAC.00263-19. PMID: 31307986; PMCID: PMC6761495.
- Turtle L, Hope W. Flucytosine (5-Fluorocytosine; 5-FC). InKucers’ The Use of Antibiotics 2017 Oct 2; 2919-2926.
- Nett JE, Andes DR. Antifungal Agents: Spectrum of Activity, Pharmacology, and Clinical Indications. Infect Dis Clin North Am. 2016 Mar;30(1):51-83. doi: 10.1016/j.idc.2015.10.012. Epub 2015 Dec 29. PMID: 26739608.
- Sleeper M. Equine cardiovascular clinical pharmacology. Equine Pharmacology. 2014 Sep 3:279.
- Chang YL, Yu SJ, Heitman J, Wellington M, Chen YL. New facets of antifungal therapy. Virulence. 2017 Feb 17;8(2):222-236. doi: 10.1080/21505594.2016.1257457. Epub 2016 Nov 7. PMID: 27820668; PMCID: PMC5354158.
- Spernovasilis N, Kofteridis DP. Pre-existing liver disease and toxicity of antifungals. J Fungi. 2018 Dec;4(4):133.
- Chastain DB, Franco-Paredes C, Stover KR. Addressing Antiretroviral Therapy-Associated Drug-Drug Interactions in Patients Requiring Treatment for Opportunistic Infections in Low-Income and Resource-Limited Settings. J Clin Pharmacol. 2017 Nov;57(11):1387-1399. doi: 10.1002/jcph.978. Epub 2017 Sep 8. PMID: 28884831.
- Shafiei M, Peyton L, Hashemzadeh M, Foroumadi A. History of the development of antifungal azoles: A review on structures, SAR, and mechanism of action. Bioorg Chem. 2020 Nov;104:104240. doi: 10.1016/j.bioorg.2020.104240. Epub 2020 Aug 28. PMID: 32906036.
- Gowda DV, Afrasim M, Meenakshi SI, Manohar M, Hemalatha S, Siddaramaiah H, Sathishbabu P, Rizvi SMD, Hussain T, Kamal MA. A Paradigm Shift in the Development of Anti-Candida Drugs. Curr Top Med Chem. 2019;19(28):2610-2628. doi: 10.2174/1568026619666191029145209. PMID: 31663480.
- Class IP, USPC A. Patent application title: COMPOSITIONS AND METHODS FOR TREATING FUNGAL INFECTIONS Inventors: James J. Collins (Newton, MA, US) James J. Collins (Newton, MA, US) Peter A. Belenky (Framingham, MA, US) Diogo M. Camacho (Framingham, MA, US).
- Nguyen Van Kinh MD, Le T, Cuc NT, Thuy PT, Hanh DT, Phuc PH, Chinh NT, Chi NH, Quang VM, Lam NT, Thuy CT. A Randomized, Open-Label, Comparative Study of the Effectiveness of Itraconazole versus Amphotericin B in the Induction Treatment of Penicilliosis in HIV-Infected Adults.
- Choi FD, Juhasz MLW, Atanaskova Mesinkovska N. Topical ketoconazole: a systematic review of current dermatological applications and future developments. J Dermatolog Treat. 2019 Dec;30(8):760-771. doi: 10.1080/09546634.2019.1573309. Epub 2019 Feb 14. PMID: 30668185.
- Chew SY, Than LT. Vulvovaginal candidosis: contemporary challenges and the future of prophylactic and therapeutic approaches. Mycoses. 2016 May;59(5):262-73. doi: 10.1111/myc.12455. Epub 2016 Jan 13. PMID: 26765516.
- Richards JS, JD R. Overview of herbal supplements. Elite Continuing Education. 2013:46-62.
- Benitez LL, Carver PL. Adverse Effects Associated with Long-Term Administration of Azole Antifungal Agents. Drugs. 2019 Jun;79(8):833-853. doi: 10.1007/s40265-019-01127-8. PMID: 31093949.
- Richards JS, JD R. Overview of herbal supplements. Elite Continuing Education. 2013:46-62.
- Bailie GR. Med facts pocket guide of Drug Interactions.
- Baxter K, Preston CL, editors. Stockley's drug interactions. London: Pharmaceutical Press; 2010 Jul.
- Mutalik M, Sanghavi D. Review of drug interactions: a comprehensive update. Journal of Pharmaceutical Research International. 2014 Mar 12:954-80.
- Shafiei M, Peyton L, Hashemzadeh M, Foroumadi A. History of the development of antifungal azoles: A review on structures, SAR, and mechanism of action. Bioorg Chem. 2020 Nov;104:104240. doi: 10.1016/j.bioorg.2020.104240. Epub 2020 Aug 28. PMID: 32906036.
- Lakhani P, Patil A, Majumdar S. Challenges in the Polyene- and Azole-Based Pharmacotherapy of Ocular Fungal Infections. J Ocul Pharmacol Ther. 2019 Jan/Feb;35(1):6-22. doi: 10.1089/jop.2018.0089. Epub 2018 Nov 8. PMID: 30481082; PMCID: PMC6354613.
- Rauseo AM, Coler-Reilly A, Larson L, Spec A. Hope on the Horizon: Novel Fungal Treatments in Development. Open Forum Infect Dis. 2020 Jan 12;7(2):ofaa016. doi: 10.1093/ofid/ofaa016. PMID: 32099843; PMCID: PMC7031074.
- Gupta AK, Stec N, Summerbell RC, Shear NH, Piguet V, Tosti A, Piraccini BM. Onychomycosis: a review. J Eur Acad Dermatol Venereol. 2020 Sep;34(9):1972-1990. doi: 10.1111/jdv.16394. Epub 2020 Jun 5. PMID: 32239567.
- Al-Kurjiya DA, Gheorghe I, Popa M, Mihaescu G, Marutescu L. Characterization of non-albicans Candida species involved in human infections. Rom Biotechnol Lett. 2019 Sep 1;24(5):837-44.
- AL-Kurjiya DA, Gheorghe I, Popa M, Mihaescu G, Marutescu L. Characterization of non-albicans Candida.
- Wilson DT, Dimondi VP, Johnson SW, Jones TM, Drew RH. Role of isavuconazole in the treatment of invasive fungal infections. Ther Clin Risk Manag. 2016 Aug 3;12:1197-206. doi: 10.2147/TCRM.S90335. PMID: 27536124; PMCID: PMC4977098.
- Schmiedel Y, Zimmerli S. Common invasive fungal diseases: an overview of invasive candidiasis, aspergillosis, cryptococcosis, and Pneumocystis pneumonia. Swiss Med Wkly. 2016 Feb 22;146:w14281. doi: 10.4414/smw.2016.14281. PMID: 26901377.
- McKeny PT, Nessel TA, Zito PM. Antifungal antibiotics. StatPearls [Internet]. 2020 Sep 29.
- Nivoix Y, Ledoux MP, Herbrecht R. Antifungal Therapy: New and Evolving Therapies. Semin Respir Crit Care Med. 2020 Feb;41(1):158-174. doi: 10.1055/s-0039-3400291. Epub 2020 Jan 30. PMID: 32000291.
- Dymond AW, So K, Martin P, Huang Y, Severin P, Mathews D, Lisbon E, Mariani G. Effects of cytochrome P450 (CYP3A4 and CYP2C19) inhibition and induction on the exposure of selumetinib, a MEK1/2 inhibitor, in healthy subjects: results from two clinical trials. Eur J Clin Pharmacol. 2017 Feb;73(2):175-184. doi: 10.1007/s00228-016-2153-7. Epub 2016 Nov 26. PMID: 27889832; PMCID: PMC5226997.
- Dzintars K, Toman LP. Interpretation and Understanding of Clinical Drug Interactions Between Azoles and Immunosuppressants in Solid Organ Transplant Recipients. Current Fungal Infection Reports. 2020 Aug 6:1-6.
- Dzintars K, Toman LP. Interpretation and Understanding of Clinical Drug Interactions Between Azoles and Immunosuppressants in Solid Organ Transplant Recipients. Current Fungal Infection Reports. 2020 Aug 6:1-6.
- Wiesner A, Zwolińska-Wcisło M, Paśko P. Effect of Food and Dosing Regimen on Safety and Efficacy of Proton Pump Inhibitors Therapy-A Literature Review. Int J Environ Res Public Health. 2021 Mar 29;18(7):3527. doi: 10.3390/ijerph18073527. PMID: 33805341; PMCID: PMC8036504.
- Dodd S, Kollipara S, Sanchez-Felix M, Kim H, Meng Q, Beato S, Heimbach T. Prediction of ARA/PPI Drug-Drug Interactions at the Drug Discovery and Development Interface. J Pharm Sci. 2019 Jan;108(1):87-101. doi: 10.1016/j.xphs.2018.10.032. Epub 2018 Oct 29. PMID: 30385285.
- Zhou H, Zhu H. Water-insoluble drugs and their pharmacokinetic behaviors. InWater-Insoluble Drug Formulation 2018 Mar 12;97-111.
- Barbosa FD. Aminothioxanthone derivatives: synthesis and study of antifungal synergistic effects.
- Chen J, He ZM, Wang FL, Zhang ZS, Liu XZ, Zhai DD, Chen WD. Curcumin and its promise as an anticancer drug: An analysis of its anticancer and antifungal effects in cancer and associated complications from invasive fungal infections. Eur J Pharmacol. 2016 Feb 5;772:33-42. doi: 10.1016/j.ejphar.2015.12.038. Epub 2015 Dec 23. PMID: 26723514.
- Seyedmousavi S, Wiederhold NP, Ebel F, Hedayati MT, Rafati H, Verweij PE. Antifungal use in veterinary practice and emergence of resistance. InEmerging and Epizootic Fungal Infections in Animals 2018;359-402.
- Seyedmousavi S, Rafati H, Ilkit M, Tolooe A, Hedayati MT, Verweij P. Systemic Antifungal Agents: Current Status and Projected Future Developments. Methods Mol Biol. 2017;1508:107-139. doi: 10.1007/978-1-4939-6515-1_5. PMID: 27837500.
- Nett JE, Andes DR. Itraconazole. InKucers’ The Use of Antibiotics 2017 Oct 2;2786-2811.
- Rehman K, Kamran SH, Akash MS. Toxicity of antibiotics. InAntibiotics and Antimicrobial Resistance Genes in the Environment 2020 Jan 1;234-252.
- Yunihastuti E, Widhani A, Karjadi TH. Drug hypersensitivity in human immunodeficiency virus-infected patient: challenging diagnosis and management. Asia Pac Allergy. 2014 Jan;4(1):54-67. doi: 10.5415/apallergy.2014.4.1.54. Epub 2014 Jan 31. PMID: 24527412; PMCID: PMC3921866.
- Megías-Vericat JE, Solana-Altabella A, Ballesta-López O, Martínez-Cuadrón D, Montesinos P. Drug-drug interactions of newly approved small molecule inhibitors for acute myeloid leukemia. Ann Hematol. 2020 Sep;99(9):1989-2007. doi: 10.1007/s00277-020-04186-0. Epub 2020 Jul 18. PMID: 32683457.
- Rogala BG, Charpentier MM, Nguyen MK, Landolf KM, Hamad L, Gaertner KM. Oral Anticancer Therapy: Management of Drug Interactions. J Oncol Pract. 2019 Feb;15(2):81-90. doi: 10.1200/JOP.18.00483. PMID: 30763198.
- Roncato R, Angelini J, Pani A, Cecchin E, Sartore-Bianchi A, Siena S, De Mattia E, Scaglione F, Toffoli G. CDK4/6 Inhibitors in Breast Cancer Treatment: Potential Interactions with Drug, Gene, and Pathophysiological Conditions. Int J Mol Sci. 2020 Sep 1;21(17):6350. doi: 10.3390/ijms21176350. PMID: 32883002; PMCID: PMC7504705.
- Ruiz ME, Montoto SS. Routes of drug administration. InADME Processes in Pharmaceutical Sciences 2018;97-133.
- Rivosecchi RM, Samanta P, Demehin M, Nguyen MH. Pharmacokinetics of Azole Antifungals in Cystic Fibrosis. Mycopathologia. 2018 Feb;183(1):139-150. doi: 10.1007/s11046-017-0189-6. Epub 2017 Aug 9. PMID: 28795298.
- Wu AY, Tseng HK, Liu CP. Prophylactic Strategies for Invasive Fungal Diseases in Hematological Stem Cell Transplantation: An Update. 2018 Feb 1;29(1):38-45.
- Nett JE, Andes DR. Antifungal Agents: Spectrum of Activity, Pharmacology, and Clinical Indications. Infect Dis Clin North Am. 2016 Mar;30(1):51-83. doi: 10.1016/j.idc.2015.10.012. Epub 2015 Dec 29. PMID: 26739608.
- Lat A, Thompson GR 3rd. Update on the optimal use of voriconazole for invasive fungal infections. Infect Drug Resist. 2011;4:43-53. doi: 10.2147/IDR.S12714. Epub 2011 Feb 3. PMID: 21694908; PMCID: PMC3108750.
- Zuckerman JN, Brunette G, Leggat P. Essential travel medicine. John Wiley & Sons; 2015 Jul 20.
- Singh B. THE Troubling benzene ring–Its impact on health, crops, drug development, environmental policy-making and the therapeutic challenge.
- Roy H, Nandi S. In-Silico Modeling in Drug Metabolism and Interaction: Current Strategies of Lead Discovery. Curr Pharm Des. 2019;25(31):3292-3305. doi: 10.2174/1381612825666190903155935. PMID: 31481001.
- Jović Z, Janković SM, Ružić Zečević D, Milovanović D, Stefanović S, Folić M, Milovanović J, Kostić M. Clinical Pharmacokinetics of Second-Generation Triazoles for the Treatment of Invasive Aspergillosis and Candidiasis. Eur J Drug Metab Pharmacokinet. 2019 Apr;44(2):139-157. doi: 10.1007/s13318-018-0513-7. PMID: 30284178.
- Amsden JR, Gubbins PO. Pharmacogenomics of triazole antifungal agents: implications for safety, tolerability and efficacy. Expert Opin Drug Metab Toxicol. 2017 Nov;13(11):1135-1146. doi: 10.1080/17425255.2017.1391213. Epub 2017 Oct 20. PMID: 29022838.
- Bahar MA, Setiawan D, Hak E, Wilffert B. Pharmacogenetics of drug-drug interaction and drug-drug-gene interaction: a systematic review on CYP2C9, CYP2C19 and CYP2D6. Pharmacogenomics. 2017 May;18(7):701-739. doi: 10.2217/pgs-2017-0194. Epub 2017 May 8. PMID: 28480783.
- Alcazar-Fuoli L, Mellado E. Ergosterol biosynthesis in Aspergillus fumigatus: its relevance as an antifungal target and role in antifungal drug resistance. Front Microbiol. 2013 Jan 10;3:439. doi: 10.3389/fmicb.2012.00439. PMID: 23335918; PMCID: PMC3541703.
- Gupta AK, Versteeg SG, Shear NH. Common drug-drug interactions in antifungal treatments for superficial fungal infections. Expert Opin Drug Metab Toxicol. 2018 Apr;14(4):387-398. doi: 10.1080/17425255.2018.1461834. Epub 2018 Apr 26. PMID: 29633864.
- Hall GS, Sekeres JA, Neuner E, Hall JO. Antifungal Agents. InInteractions of Yeasts, Moulds, and Antifungal Agents 2012;1-64.
- Gupta AK, FOLEY KA. Systemic antifungal agents. Comprehensive dermatologic drug therapy. 2012 Oct 18;3:98-120.
- Petersen AB, Rønnest MH, Larsen TO, Clausen MH. The chemistry of griseofulvin. Chem Rev. 2014 Dec 24;114(24):12088-107. doi: 10.1021/cr400368e. Epub 2014 Dec 5. PMID: 25476923.
- Leung AK, Lam JM, Leong KF, Hon KL. Tinea corporis: an updated review. Drugs Context. 2020 Jul 20;9:2020-5-6. doi: 10.7573/dic.2020-5-6. PMID: 32742295; PMCID: PMC7375854.
- Buckley D. Fungal and Yeast Infection of Skin, Hair and Nails. InTextbook of Primary Care Dermatology 2021;243-249.
- Kathiravan MK, Salake AB, Chothe AS, Dudhe PB, Watode RP, Mukta MS, Gadhwe S. The biology and chemistry of antifungal agents: a review. Bioorg Med Chem. 2012 Oct 1;20(19):5678-98. doi: 10.1016/j.bmc.2012.04.045. Epub 2012 May 9. Erratum in: Bioorg Med Chem. 2013 Feb 1;21(3):834. Erratum in: Bioorg Med Chem. 2013 Mar 1;21(5):1367. PMID: 22902032.