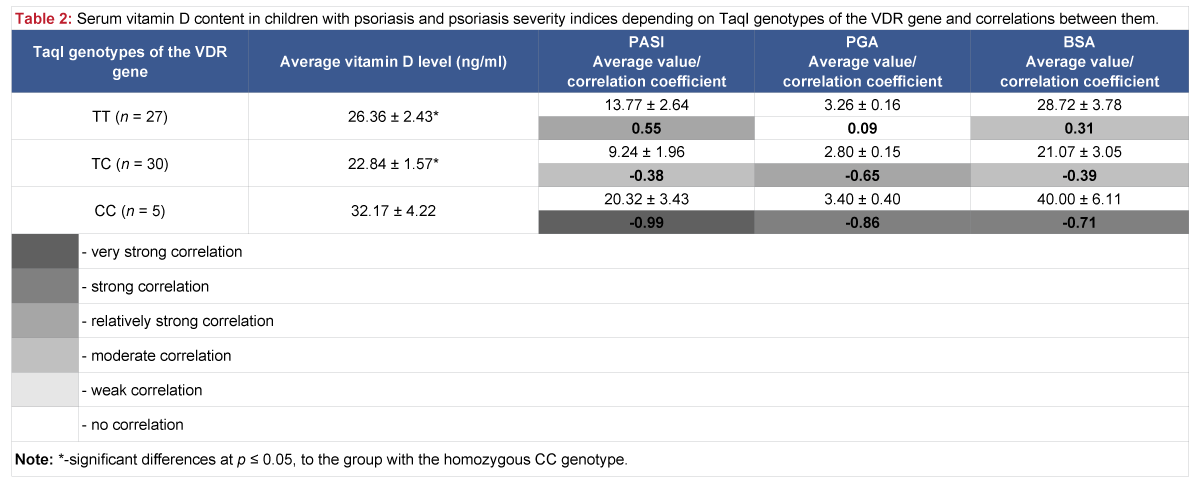More Information
Submitted: March 29, 2021 | Approved: April 06, 2021 | Published: April 07, 2021
How to cite this article: Elvina M. Clinical and epidemiological differences in the course of psoriasis in children depending on Vitamin D levels and genotypes of the TaqI polymorphic variant of the VDR gene. Ann Dermatol Res. 2021; 5: 006-012.
DOI: 10.29328/journal.adr.1001014
ORCiD: orcid.org/0000–0002–3440–0745
Copyright License: © 2021 Elvina M. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Psoriasis; Children with psoriasis; TT; TC and CC TaqI (T/C) genotypes of the VDR gene; Vitamin D serum level; PASI; PGA and BSA indices of psoriasis severity
Clinical and epidemiological differences in the course of psoriasis in children depending on Vitamin D levels and genotypes of the TaqI polymorphic variant of the VDR gene
Murzina Elvina*
Department of Dermatovenereology, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine
*Address for Correspondence: Murzina Elvina, PhD, Associate Professor of the Department of Dermatovenereology, Shupyk National Medical Academy of Postgraduate Education, 9 Dorohozhytska Str., Kyiv, 04112 Ukraine, Tel: +380677355756; Email: [email protected]
When grouping children with psoriasis depending on TaqI (T/C) genotypes of the VDR gene, the youngest age of disease onset and the longest duration of dermatitis (5.60 ± 0.77 years and 4.90 ± 0.68 years, respectively) showed up in case of the CC genotype. In case of the TT genotype, disease onset coincided with an older age, and the history of present illness was the shortest (10.26 ± 0.64 years and 2.59 ± 0.58 years, respectively). PASI (20.32 ± 3.43) and BSA (40.00 ± 6.11) severity indices were the highest and of statistically significant difference to those in other groups in the presence of the CC genotype. In case of the TC genotype, the index PGA (2.80 ± 0.15) was the lowest and made a statistically significant difference to the values of other groups. A negative correlation between vitamin D levels and the PASI, PGA, BSA was identified in children holding CC and TC genotypes.
Conclusion: The clinical presentation of dermatitis and its epidemiological features in children with psoriasis, namely the age of disease onset, duration of exacerbation, body surface area and the intensity of psoriasis symptoms depend on vitamin D serum levels and genotypes of the TaqI polymorphic variant of the VDR gene.
Vitamin D function is mediated by means of receptors (Vitamin D receptor – VDR), represented in all body organs and tissues [1]. VDR fulfills the active role in the transcription of various genes responsible for controlling the cell cycle, apoptosis, and have few polymorphisms [2,3]. The human VDR gene was mapped on the chromosome 12 and contains four common polymorphisms, or to be exact, rs7975232 A/C in intron 8 (ApaI), rs1544410 G/A in intron 8 (BsmI), rs2228570 T/C in exon 2 (FokI) and rs731236 T/C in exon 9 (TaqI) [4,5]. Additionally, an imbalance exists among rs7975232, rs1544410 and rs731236 polymorphisms [6,7]. A strong correlation exists between the absence of BsmI (B allele) and the presence of TaqI sites (t allele) [8], and these sites demonstrate significant inadequate linkage with the ApaI polymorphism. However, such a linkage is not universal. In certain nationalities, Russians in particular, rs1544410 (BsmI) and rs731236 (TaqI) polymorphisms of the VDR gene basically duplicate each other, as far as both polymorphisms are closely linked and inherited together [9].
The role of polymorphic variants of the VDR gene, as a risk factor for the development of psoriasis, was studied in several populations, but is controversial. Richetta, et al. [10] revealed the A-1021G polymorphism, among widespread polymorphisms of the VDR (A-1012G, FokI, BsmI, ApaI and TaqI), to be associated with the risk of psoriasis in Italy population. In the other study the ApaI and specific haplotype of the VDR 5 polymorphism were associated with the risk of psoriasis in Chinese [11]. In contrast, the studies conducted in Croatia and Egypt identified no contribution of the VDR in psoriasis [12-14].
Meanwhile, some reports are available indicating that the VDR polymorphism also modulates the patients’ response to different treatment patterns in psoriasis in addition to increasing the risk of psoriasis. Rayan, et al. [15] revealed that the shorter period of remission was observed when treating patients with NB-UVB in case of psoriasis association with TaqI polymorphic variant of the VDR. Another studies suggested positive association of A-1012G, FokI, and TaqI alleles (wild type) of VDR polymorphic variants and the response to treating with topical calcipotriene [16,17]. A topical combination of a vitamin D3 analogue and corticosteroid is widely used for the treatment of psoriasis - a TH17-mediated disorder, which plays a major role in the development and in the disease outcomes of human autoimmune/inflammatory diseases [18,19].
The large-scale research of VDR polymorphisms and impact on pathogenesis components in psoriasis is conducted so far. However, the weight of the VDR polymorphism remains unclear, controversial, variable depending on gender, age, ethnic group, bone mass, association with parathyroid hormone, calcium effect, lifestyle and health status [20]. Distinctions between the studies on genetic risks of various diseases may be explained by genetic heterogeneity, genetic pollution by population criterion, interactions between genes and their environment [21].
Aims
To compare epidemiological and clinical features of psoriasis in children depending on TaqI (T/C) genotypes of the VDR gene and Vitamin D levels.
We examined 62 children with psoriasis, 22 boys and 33 girls aged 4-17, the mean age – 12.30 ± 0.45 years, who underwent treatment in Kyiv Municipal Dermatology-STI Clinic. The ratio between girls and boys came to 1.48: 25 boys and 37 girls that coincide with the findings of multicenter cross-sectional study in the US [22].
According to the guidelines by the U.S. National Psoriasis Foundation the severity of psoriasis in children is assessed on the BSA (Body Surface Area) index – total body surface area affected by the disease process; 1% of the body surface area is matching with the palm surface area of a patient: mild psoriasis is diagnosed when the BSA<3, moderate 3
We also calculated the PASI index (Psoriasis Area and Severity Index) - an index for the extent of body surface involvement and severity of psoriasis - reflects the affected body surface area, taking into account the intensity of signs such as erythema, exfoliation and infiltration, the score obtained ranges from 0 to 72 points; and the I/PGA (Investigator/Physician’s Global Assessment Scale) index or PGA is the index of the severity of psoriasis that reflects disease intensity [25].
We genotyped children with psoriasis in order to determine Taql (T/C) polymorphic variant of the VDR gene. The buccal epithelium, taken from children using sterile brushes, packaged in sterile Eppendorf tubes served as the material for genotyping. The material was stored at +4 °С. Specimens were transported to the lab by maintaining the cold chain environment. Isolation of DNA from buccal epithelial cells and determination of the VDR TaqI polymorphic variant were performed using polymerase chain reaction (PCR) technique and the following restriction fragment length polymorphism (RFLP) [26].
We tested vitamin D serum levels, namely 25(OH)D, in the accredited laboratory. Vitamin D serum levels at 30-60 ng/ml were considered as optimal, 20-30 ng/ml - insufficient, less than 20 ng/ml - deficiency. Study records were processed by means of statistical methods of parametric and non-parametric tests using the application STATISTICA 13.3 (developer –Stat Soft. Inc). The Student’s t-test, Fisher criterion, Mann-Whitney U test and the Pearson’s chi-squared test were used when comparing the mean values of data sets, with the level of statistical significance p ≤ 0.05. A correlation analysis was conducted by applying the Pearson’s correlation coefficient. The values of the correlation coefficient (r) were interpreted according to the Chaddock scale.
Psoriasis was determined based on clinical findings and generally accepted diagnostic criteria. In 22 (35.48%) psoriasis was recognized for the first time. Disease onset averaged out at 8.84 ± 0.51 years. A disease duration ranged from several months to 10 years, 3.38 ± 0.48 years on average. The duration of the last exacerbation in certain children coincided with disease onset and ranged from 2 weeks to 2 years, 11.85 ± 2.44 weeks on average.
Types by presentation of the disease mostly involved plaque psoriasis – 42 (67.74%), guttate psoriasis was recognized in 8 (12.90%) children, inverse psoriasis – in 6 (9.68%) children, scalp psoriasis – in 4 (6.45%) children and palmoplantar type – in 2 (3.23%) children.
The assessment of psoriasis severity in children showed that in the majority of children, 43 (69.35%), BSA was higher than 10 points and provided the average BSA of 25.54 ± 2.46 that indicates the severe course of psoriasis in children according to the area affected. However, according to the average PGA index number, which comes to 3.06 ± 0.11, the overall intensity of psoriasis pathogenesis in children was rated as moderate. An in-depth analysis revealed of 24 (38.71%) children to have the PGA index being equal to 4 - severe psoriasis; moderate developed in of 19 (30.65%) children, PGA equals 3; 18 children (29.03%) have the intensity of 2 points and 1 child with PGA=1. The correlation analysis involving the child’s age and the PGA index revealed an increase in the intensity of skin lesion in psoriasis with age, the correlation coefficient – r = 0.50.
The calculated PASI index averaged out at 11.85 ± 1.44, which also rates psoriasis as moderate, however, the greatest number of children, 36 children (58.06%), had the PASI being up to 10; 13 children (20.97%) with PASI being within the range 10‑20; 8 children (12.90%) with the PASI ranging from 20 to 30 and 5 children suffered from psoriasis that was estimated on the PASI index at more than 30.
The average serum level of vitamin D in children with psoriasis averaged out at 24.95 ± 1.36 ng/ml, being considered as insufficient, although the optimal serum level of vitamin D was observed in 15 (24.19%) children. Within the deficiency range the level of serum vitamin D was reported in 21 (33.87%) children, as insufficient - in 26 (41.94%) children.
Based on the study of the VDR polymorphic variant we identified, by the frequency of TaqI (T/A) genotypes, that genotypes in children with psoriasis are mostly represented by homozygous TT and heterozygous TC variants, 27 (43.55%) and 30 (48.39%) children, respectively. Only 5 (8.06%) children were the holders of CC genotype.
When comparing epidemiological features in groups formed depending on TaqI genotypes of the VDR gene, we identified that the group of the TT genotype was represented by boys and girls almost equally, 13 and 14. The number of girls in the group with the TC genotype was higher – 18 as opposed to 12 boys. The group with the CC genotype of the TaqI polymorphic variant was represented exceptionally by girls – 5 individuals (Table 1). Supposedly, statistically significant gender differences were not detected.
| Table 1: Epidemiological characteristics of groups by TaqI genotypes of the VDR gene. | ||||||
| TaqI genotypes of the VDR gene | Gender | Mean age, years |
Mean age of disease onset, years | Average duration of the disease, years | Average duration of exacerbation, weeks | |
| M abs./% | F abs./% | |||||
| ТТ (n = 27) | 13/48.15 | 14/51.85 | 12.85 ± 0.69 | 10.26 ± 0.64 * | 2.59 ± 0.58 *** | 8.42 ± 1.21 |
| ТС (n = 30) | 12/40.00 | 18/60.00 | 11.70 ± 0.59 | 8.10 ± 0.79 */** | 3.84 ± 0.82 | 8.54 ± 1.94 |
| СС (n = 5) | 0/0 | 5/100.00 | 10.60 ± 0.90 | 5.60 ± 0.77 | 4.90 ± 0.68 | 12.00 ± 1.93 |
| χ2 = 4.067 p = 0.131 |
||||||
| Note: *-significant differences at p ≤ 0.05 in case of the CC genotype; **-significant differences at p ≤ 0.05 in case of the CC genotype; ***-significant differences at p ≤ 0.05 in case of the CC genotype | ||||||
The youngest age of children with psoriasis – in the group with homozygous CC genotype (10.6 ± 0.90 years), the oldest – with homozygous TT genotype (12.85 ± 0.69 years), whereas no significant differences depending on the age among groups were detected. Concerning the age of disease onset, it was the youngest (5.60 ± 0.77 years) in the group with the homozygous CC genotype at the level of a statistically significant difference to other groups. In case of the heterozygous TC genotype the age of disease onset came to 8.10 ± 0.79 years that was statistically significantly lower comparing to homozygous TT genotype (10.26 ± 0.64 years), although it coincided with the common tendency for psoriasis onset, or 8-11 years to be exact [27-30].
Accordingly, earlier disease onset implies a longer course, as evidenced by the relatively strong negative correlation, r = -0.60. It indicated the longest disease duration in children with homozygous CC genotype (4.90 ± 0.68 years), while in case of the homozygous TT genotype a disease duration comprised 2.59 ± 0.58 years, and their difference was statistically significant.
Psoriatic exacerbations in children ranged in duration from 1 week to 6 months. In the process of calculation we did not identify any statistically significant difference between the average duration of psoriatic exacerbation depending on groups, although resulting data reveal that the duration of psoriatic exacerbation in case of the homozygous CC genotype (12.00 ± 2.25 weeks) of the VDR TaqI polymorphic variant is considerably longer than in the heterozygous TC genotype (8.54 ± 1.94 weeks) and homozygous TT genotype (8.42 ± 1.21 weeks).
As for the homozygous CC genotype, a strong positive correlation between a child’s age, at the time of disease onset, and exacerbation duration exists, r = 0.74, as well as very strong negative correlation between exacerbation duration and disease duration, r = -0.94. It is worth noting that in children with the homozygous CC genotype, psoriasis onset at the early age is followed by more durable exacerbations, which gradually shorten with the course of the disease. At the moment of disease onset at the older age, exacerbations are of a shorter duration.
The impact of genotypes of the VDR TaqI (T/C) polymorphic variant on the symptoms of psoriasis types in children and extent of body surface involvement are not identified. Plaque psoriasis prevails in each group of children, from 60.00% in the group with the homozygous CC genotype to 83.33% in the group with the homozygous TT genotype.
To finalize the description made for clinical features of psoriasis course in the groups selected, we assessed the severity and extent of body surface involvement according to PGA, BSA, PASI indices. The findings showed that the PGA, or the severity index, indicating the intensity of the disease process, was reported the smallest in the group of children with the heterozygous TC genotype (2.80 ± 0.15), characterizing the severity as mild. In children with homozygous TT and CC genotypes, the PGA index reflected the intensity of psoriasis associated with moderate infiltration, exfoliation and erythema (3.26 ± 0.16 and 3.40 ± 0.40, respectively). Psoriasis Area and Severity Index (PASI) that indicates the area of body surface involvement, taking into account the intensity of signs such as erythema, exfoliation and infiltration, was the greatest in the group of children with the homozygous CC genotype (20.32 ± 3.43), and demonstrated a statistically significant difference to the group of children with the heterozygous genotype (9.24 ± 1.96) and was at the level of statistical trend to the homozygous TT genotype (13.77 ± 2.64). Also the BSA index, implying the body surface area, affected by the disease, was significantly higher in the group of children with the homozygous CC genotype (40.00 ± 6,11) than in other groups, although significantly differed only from the group of children with the heterozygous TC genotype (21.07 ± 3.05), and had a statistical trend to differ in the BSA index from the group of children with TT genotype (28.72 ± 3.78). It provides the opportunity to emphasize that the Body Surface Area strongly impacts the indices of severity in children with the homozygous CC genotype of the VDR TaqI polymorphic variant, and this Area exceeds the values of the Body’s Surface Area in other groups, and eventually reaches 70% of the Body Surface Area.
The analysis of vitamin D serum level in children with psoriasis, depending on genotypes of the VDR TaqI polymorphic variant, revealed that vitamin D level is considered optimal (32.17 ± 4.22 ng/ml) in the group with the homozygous CC genotype, but considerably higher than in other groups, where vitamin D level was insufficient: in case of the homozygous TT genotype – 26.36 ± 2.43 ng/ml and heterozygous TC genotype – 22.84 ± 1.57 ng/ml (Table 2).
Table 2: Serum vitamin D content in children with psoriasis and psoriasis severity indices depending on TaqI genotypes of the VDR gene and correlations between them.
When analyzing interrelations between vitamin D serum levels in children and characteristics of the disease, the dependence of psoriasis severity indices on vitamin D levels was determined. Particularly, in the group with the homozygous CC genotype of the TaqI polymorphic variant, where the correlation between vitamin D levels and the intensity of the disease process, as well as the area affected (PASI, PGA, BSA), is very strong negative and strong negative (r = -0.99, r = ‑ 0.86, r = -0.71, respectively). Moderate negative correlations between vitamin D levels and PASI and BSA indices also exist in the group of children with the heterozygous TC genotype, r = -0.38 and r = -0.39, and relatively strong negative correlation between vitamin D levels and the PGA index, r = -0.65.
Supposedly, existence of the above-mentioned correlations is determined by the fact that the group with the homozygous CC genotype is represented by girls only, while in the group with the heterozygous variant girls just outnumber. Therefore, we conducted the analysis of correlations between serum vitamin D levels in children and disease symptoms depending on gender – children’s groups with the homozygous TT and heterozygous TC genotypes were divided into groups including girls and boys (Table 3).
| Table 3: Epidemiological features of psoriasis in groups with TaqI genotypes of the VDR gene depending on gender. | |||||
| TaqI genotypes of the VDR gene |
Gender | Mean age, years |
Mean age of disease onset, years |
Average duration of the disease, years |
Average duration of exacerbation, weeks |
| ТТ (n = 27) | boys (n = 13) | 12.69 ± 1.04 | 10.00 ± 1.02 | 2.76 ± 1.06 | 9.31 ± 1.87 |
| girls (n = 14) | 13.00 ± 0.96 | 10.50 ± 0.82 | 2.42 ± 0.58 | 7.54 ± 1.56 | |
| ТС (n = 30) | boys (n = 12) | 13.75 ± 0.81* | 9.08 ± 1.32 | 4.94 ± 0.97 | 5.09 ± 0.77** |
| girls (n = 18) | 10.33 ± 0.67* | 7.44 ± 0.99 | 3.10 ± 0.88 | 10.64 ± 3.02** | |
| Note: *-significant differences at p ≤ 0.05; **-significant differences at p ≤ 0.1 | |||||
When studying the characteristics of groups it was found that groups were compared by the age of disease onset, average disease duration. A statistically significant difference by the mean age between groups of boys and girls with the heterozygous TC genotype exists.
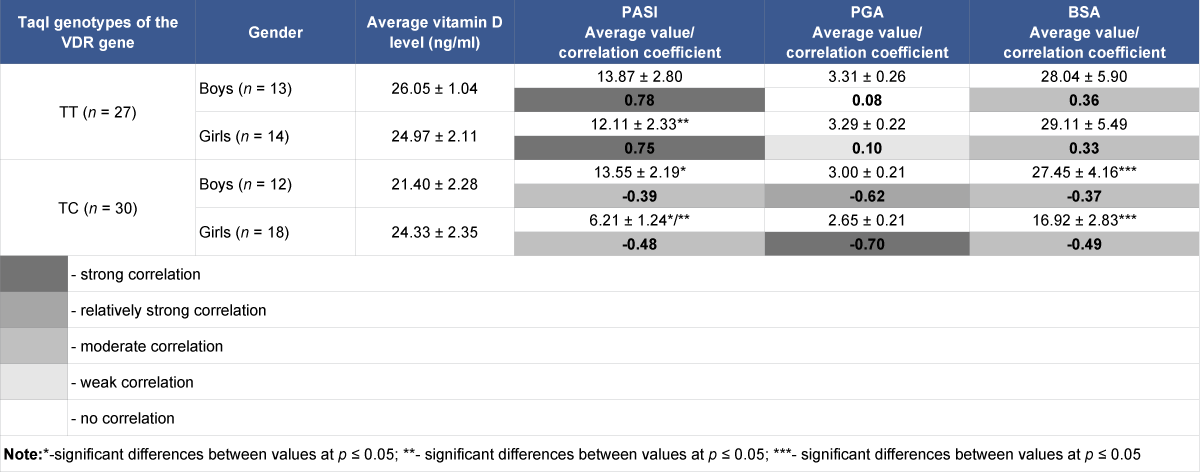
Depending on gender, there is no statistically significant difference in vitamin D serum levels in girls and boys both in the group with the homozygous TT genotype and heterozygous TC genotype (Table 4).
Table 4: Serum vitamin D content in children with psoriasis and severity indices depending on gender and TaqI genotypes of the VDR gene, and correlations between values.
When calculating and comparing average PASI, PGA, BSA indices between groups of girls and boys depending on the genotype of the TaqI polymorphic variant, a statistically significant difference between average PASI and BSA indices between groups of girls and boys with heterozygous genotypes was detected. Also a statistically significant difference of average PASI, PGA, BSA indices between group of girls with heterozygous TC genotype and groups of girls and boys with homozygous TT genotype was detected.
In case of the heterozygous TC genotype, negative correlations exist between serum vitamin D levels and severity indices (PASI, PGA, BSA), which in the group of girls tend to be larger than in the boys’ group, moderate and strong (r = -0.48, r = -0.70, r = -0.49, respectively), in the boys’ group, moderate and relatively strong (r = -0.39, r = -0.62, r = -0.37, respectively). In contrast, completely different correlations were found between vitamin D levels and severity indices in the groups of girls and boys with homozygous genotypes ТТ, with PASI severity index – strong positive (0,75 and 0,78, respectively), with BSA severity index – moderate positive (0,33 and 0,36).
When comparing the findings in our study with the findings of others, it is worth noting that the significance of the VDR polymorphism remains controversial and varies from one study to another. The distribution of genotypes of the TaqI polymorphic variant, we have got, complies with data of the studies involving Indian population, when the homozygous TT genotype was detected in 49%, while the heterozygous TC genotype – in 43% and homozygous CC in 8% [21].
The comparison of epidemiological and clinical features of psoriasis in the presence of different genotypes of the TaqI polymorphic variant offered evidences of TT and TC genotypes to be characterized by psoriasis onset at the common age of 9-11 years, while in case of the CC genotype in girls psoriasis develops early, coinciding with 5-6 years, and has the longest course (4.90 ± 0.79 years). This genotype is also characterized by more durable exacerbations of psoriasis, associated with the age of onset and duration of the disease process - the older the child at the time of disease onset and the shorter the disease duration, the longer the exacerbation of psoriasis lasts, and the disease process affects the greater body surface area, which is proved by a supposedly higher PASI index (20.32 ± 3.43) and the highest BSA index (40.00 ± 6.11).
Due to the validation of the results of numerous studies, which assert that the deficiency and insufficient level of serum vitamin D in patients with psoriasis plays a significant role [31–33] and identified a negative correlation between 25(OH)D levels and the severity of psoriasis [34–38], we identified a relationship between the severity of psoriasis and vitamin D serum levels in children with CC and TC genotypes of the VDR TaqI polymorphic variant: a decrease in vitamin D levels led to an increase in the severity of dermatitis, due to the greater extent of body surface involvement and higher intensity of erythema, exfoliation and infiltration.
It turns out that in children with the homozygous TT genotype of the TaqI polymorphic variant, an increase in serum vitamin D levels will lead to an increase in the severity of the disease, or it can indicate the resistance of dermal fibroblasts to vitamin D and resistance to treatment with calcipotriol, as Turkish researchers heeded [39].
The lack of absolute distinctions in VDR genotypes between patients or children with psoriasis and healthy controls proved heterogeneity of the causes of psoriasis. Other factors exist that contribute to these distinctions: the relationship between alleles of the VDR gene and other genes, external environment, lifestyle.
Determining the genotypes of the TaqI polymorphic variant of the VDR gene enables to choose the most effective treatment of children with psoriasis, avoid wasting time and family finances on drugs to which the pathological process is not sensitive.
The clinical presentation of dermatitis and its epidemiological features in children with psoriasis, namely the age of disease onset, duration of exacerbation, body surface area and the intensity of psoriasis symptoms depend on vitamin D serum levels and genotypes of the TaqI polymorphic variant of the VDR gene.
- Welsh JE, Wietzke JA, Zinser G, Byrne B, Smith K, et al. Vitamin D-3 Receptor as a Target for Breast Cancer Prevention. J Nutr. 2003; 133: 2425-2433. PubMed: https://pubmed.ncbi.nlm.nih.gov/12840219/
- Pike JW, Meyers M, Watanuki M, Kim S, Zella LA, et al. Perspectives on mechanisms of gene regulation by 1,25-dihydroxyvitamin D3 and its receptor. J Steroid Biochem Mol Biol. 2007; 103: 389-395. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1868541/
- Rukin NJ, Strange RC. What are the frequency, distribution and functional effects of vitamin D receptor polymorphisms as related to cancer risk? Nutr Rev. 2007; 65: 96-101. PubMed: https://pubmed.ncbi.nlm.nih.gov/17867380/
- Uitterlinden AG, Fang Y, Van Meurs JBJ, Pols HAP, Van Leeuwen JPTM, et al. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004; 2338: 143-156. PubMed: https://pubmed.ncbi.nlm.nih.gov/15315818/
- Khan MI, Bielecka ZF, Najm MZ, Bartnik E, Czarnecki JS, et al. Vitamin D receptor gene polymorphisms in breast and renal cancer: Current state and future approaches (Review). Int J Oncol. 2014; 44: 349-363. PubMed: https://pubmed.ncbi.nlm.nih.gov/24297042/
- Kostner K, Denzer N, Muller CS, Klein R, Tilgen W, et al. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res. 2009; 29: 3511–3536. PubMed: https://pubmed.ncbi.nlm.nih.gov/19667145/
- Labuda M, Fujiwara TM, Ross MV, Morgan K, Garcia-Heras J, et al. Two hereditary defects related to vitamin D metabolism map to the same region of human chromosome 12q13-14. J Bone Miner Res. 1992; 7: 1447-1453. PubMed: https://pubmed.ncbi.nlm.nih.gov/1336301/
- Bid HK, Mishra DK, Mittal RD, Vitamin-D Receptor (VDR) Gene (Fok-I, Taq-I & Apa-I) Polymorphisms in Healthy Individuals from North Indian Population. APJCP. 2005; 6: 147-152. PubMed: https://pubmed.ncbi.nlm.nih.gov/16101324/
- Klimova OYU, Berdnikova NG, Kazakov RE. Pleiotropic effects of vitamin D: an essential element of comorbidity therapy. Consilium Medicum. 2017; 19: 114–121.
- Richetta AG, Silvestri V, Giancristoforo S, Rizzolo P, D'Epiro S, et al. A-1012G promoter polymorphism of vitamin D receptor gene is associated with psoriasis risk and lower allele-specific expression. DNA Cell Biol. 2014; 33: 102–109. PubMed: https://pubmed.ncbi.nlm.nih.gov/24320988/
- Zhou X, Xu LD, Li YZ. The association of polymorphisms of the vitamin D receptor gene with psoriasis in the Han population of northeastern China. J Dermatol Sci. 2014; 73: 63–66. PubMed: https://pubmed.ncbi.nlm.nih.gov/24055231/
- Polic MV, Rucevic I, Barisic-Drusko V, Miskulin M, Glavas-Obrovac L, et al. Polymorphisms of vitamin D receptor gene in the population of eastern Croatia with psoriasis vulgaris and diabetes mellitus. Coll Antropol. 2012; 36: 451–457. PubMed: https://pubmed.ncbi.nlm.nih.gov/22856230/
- Rucevic I, Stefanic M, Tokic S, Vuksic M, Glavas-Obrovac L, et al. Lack of association of vitamin D receptor gene 3′-haplotypes with psoriasis in Croatian patients. J Dermatol. 2012; 39: 58–62. PubMed: https://pubmed.ncbi.nlm.nih.gov/21951018/
- Zuel-Fakkar NM, Kamel MM, Asaad MK, Mahran MZ, Shehab AA. A study of ApaI and TaqI genotypes of the vitamin D receptor in Egyptian patients with psoriasis. Clin Exp Dermatol. 2011; 36: 355–359. PubMed: https://pubmed.ncbi.nlm.nih.gov/21198789/
- Ryan C, Renfro L, Collins P, Kirby B, Rogers S. Clinical and genetic predictors of response to narrowband ultraviolet B for the treatment of chronic plaque psoriasis. Br J Dermatol. 2010; 163: 1056–1063. PubMed: https://pubmed.ncbi.nlm.nih.gov/20716226/
- Halsall JA, Osborne JE, Pringle JH, Hutchinson PE. Vitamin D receptor gene polymorphisms, particularly the novel A-1012G promoter polymorphism, are associated with vitamin D3 responsiveness and non-familial susceptibility in psoriasis. Pharmacogenet Genomics. 2005; 15: 349–355. PubMed: https://pubmed.ncbi.nlm.nih.gov/15864137/
- Saeki H, Asano N, Tsunemi Y, Takekoshi T, Kishimoto M, et al. Polymorphisms of vitamin D receptor gene in Japanese patients with psoriasis vulgaris. J Dermatol Sci. 2002; 30: 167–171. PubMed: https://pubmed.ncbi.nlm.nih.gov/12413773/
- Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014; 13: 668-677. PubMed: https://pubmed.ncbi.nlm.nih.gov/24418308/
- Fujiyama T, Ito T, Umayahara T, Ikeya S, Tatsuno K, et al. Topical application of a vitamin D3 analogue and corticosteroid to psoriasis plaques decreases skin infiltration of TH17 cells and their ex vivo expansion/ J Allergy Clin Immunol. 2016; 138: 517-528. PubMed: https://pubmed.ncbi.nlm.nih.gov/27315769/
- Yakovleva OA, Nikolova OM, Doroshkevych IA, Shcherbeniuk NV. Genetic polymorphism of vitamin D receptor determines its metabolism and efficiency. Pain joints spine. 2017; 7: 73-78.
- Bhanushali AA, Lajpal N, Kulkarni SS, Chavan SS, Bagadi SS, et al. Das Frequency of fokI and taqI polymorphism of vitamin D receptor gene in Indian population and its association with 25-hydroxyvitamin D levels. Indian J Hum Genet. 2009; 15: 108–113. PubMed: https://pubmed.ncbi.nlm.nih.gov/21088715/
- Mercy K, Kwasny M, Cordoro KM, Menter A, Tom WL, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatr Dermatol. 2013; 30: 424–428. PubMed: https://pubmed.ncbi.nlm.nih.gov/23360462/
- Menter A, Cordoro KM, Davis DMR, Kroshinsky D, Paller AS, et al. Joint American Academy of Dermatology National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020; 82: 161-201. PubMed: https://pubmed.ncbi.nlm.nih.gov/31703821/
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. https://www.jaad.org/article/S0190-9622(20)32288-X/fulltext
- Bożek A, Reich A. The reliability of three psoriasis assessment tools: Psoriasis area and severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017; 26: 851–856. PubMed: https://pubmed.ncbi.nlm.nih.gov/29068583/
- Berezenko VS, Mykhailiuk KZ, Rossokha ZІ, Kyriachenko SP. Correlation of polymorphous variants (ApaI, TagI, BsmI) of the VDR receptor gene with the vitamin D level and liver fibrosis in children with autoimmune hepatitis. Zaporozhye Med J. 2019; 21: 458-465.
- Kwon HH, Na SJ, Jo SJ, Youn JI. Epidemiology and clinical features of pediatric psoriasis in tertiary referral psoriasis clinic. J Dermatol. 2012; 39: 260–264. PubMed: https://pubmed.ncbi.nlm.nih.gov/22211370/
- Seyhan M, Coskun BK, Saglam H, Ozcan H, Karincaoğlu Y. Psoriasis in childhood and adolescence: evaluation of demographic and clinical features. Pediatr Int. 2006; 48: 525–530. PubMed: https://pubmed.ncbi.nlm.nih.gov/17168968/
- Shah KN. Diagnosis and treatment of pediatric psoriasis: current and future. Am J Clin Dermatol. 2013; 14: 195–213. PubMed: https://pubmed.ncbi.nlm.nih.gov/23677694/
- Tollefson MM, Crowson CS, McEvoy MT, Maradit Kremers H. Incidence of psoriasis in children: a population-based study. J Am Acad Dermatol. 2010; 62: 979–987. PubMed: https://pubmed.ncbi.nlm.nih.gov/19962785/
- Mattozzi C, Paolino G, Salvi M, Macaluso L, Luci C, et al. Peripheral blood regulatory T cell measurements correlate with serum vitamin D level in patients with psoriasis. Eur Rev Med Pharmacol Sci. 2016; 20: 1675–1679. PubMed: https://pubmed.ncbi.nlm.nih.gov/27212156/
- Maleki M, Nahidi Y, Azizahari S, Meibodi NT, Hadianfar A. Serum 25-OH vitamin D level in psoriatic patients and comparison with control subjects. J Cutan Med Surg. 2016; 20: 207–210. PubMed: https://pubmed.ncbi.nlm.nih.gov/26654984/
- Gisondi P, Rossini M, Di Cesare A, Idolazzi L, Farina S, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012; 166: 505–510. PubMed: https://pubmed.ncbi.nlm.nih.gov/22013980/
- Chandrashekar L, Kumarit GR, Rajappa M, Revathy G, Munisamy M, et al. 25-Hydroxy vitamin D and ischaemia-modified albumin levels in psoriasis and their association with disease severity. Br J Biomed Sci. 2015; 72: 56–60. PubMed: https://pubmed.ncbi.nlm.nih.gov/26126320/
- Orgaz-Molina J, Magro-Checa C, Rosales-Alexander JL, Arrabal-Polo MA, Castellote-Caballero L, et al. Vitamin D insufficiency is associated with higher carotid intima-media thickness in psoriatic patients. Eur J Dermatol. 2014; 24: 53–62. PubMed: https://pubmed.ncbi.nlm.nih.gov/24509438/
- Al-Mutairi N, Shaaban D. Effect of narrowband ultraviolet B therapy on serum vitamin D and cathelicidin (LL-37) in patients with chronic plaque psoriasis. J Cutan Med Surg. 2014; 18: 43–48. PubMed: https://pubmed.ncbi.nlm.nih.gov/24377473/
- Atwa MA, Balata MG, Hussein AM, Abdelrahman NI, Elminshawy HH, et al. Serum 25-hydroxyvitamin D concentration in patients with psoriasis and rheumatoid arthritis and its association with disease activity and serum tumor necrosis factor-alpha. Saudi Med J. 2013; 34: 806–813. PubMed: https://pubmed.ncbi.nlm.nih.gov/23974451/
- Ricceri F, Pescitelli L, Tripo L, Prignano F. Deficiency of serum concentration of 25-hydroxyvitamin D correlates with severity of disease in chronic plaque psoriasis. J Am Acad Dermatol. 2013; 68: 511–513. PubMed: https://pubmed.ncbi.nlm.nih.gov/23394917/
- Dayangac-Erden D, Karaduman A, Erdem-Yurter H. Polymorphisms of vitamin D receptor gene in Turkish familial psoriasis patients. Arch Dermatol Res. 2007; 299: 487-491. PubMed: https://pubmed.ncbi.nlm.nih.gov/17763859/