More Information
Submitted: 23 July 2019 | Approved: 06 August 2019 | Published: 07 August 2019
How to cite this article: Discolouration, Pigmentation-Solar Lentigo. Ann Dermatol Res. 2019; 3: 001-006.
DOI: 10.29328/journal.adr.1001006
Copyright License: © 2019 Bajaj A. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Daub, Discolouration, Pigmentation-Solar Lentigo
Anubha Bajaj*
Histopathologist, AB Diagnostics, New Delhi, India
*Address for Correspondence: Anubha Bajaji, Histopathologist, AB Diagnostics, New Delhi, India, Tel: 00911141446785; Email: [email protected]; [email protected]
Preface
Solar lentigo is defined as an alteration in cutaneous pigment deposition on account of exposure to ultraviolet radiation. Solar lentigo is a benign, pigmented lesion with a characteristic increment in the quantification of pigmented keratinocytes. It can manifest as a dark brown spot on the skin.
The benign, pigmented spot or solar lentigo or multiple solar lentigines are preponderantly delineated in the sun exposed skin in a majority (> 90%) of Caucasians above 60 years of age although younger individuals and Asians can be implicated.
Solar lentigines are induced by repetitive exposure to ultraviolet light with constituent mutagenic potential. Ultraviolet radiation can induce a localized proliferation of melanocytes with a subsequent accumulation of melanin within the keratinocytes.
Individuals who are genetic carriers of one or two melanocortin-1- receptor (MC1R) gene or cogent variants demonstrate a 1.5 to twice the probability of developing solar lentigines [1,2].
Normal physiology
Cutaneous pigmentation is contingent to the presence of melanocytes which are generated from neural crest cells during embryogenesis and aptly migrate as melanoblast precursors. Native hair follicle melanoblasts differentiate into a dual cell population such as hair matrix melanocytes which engender preliminary hair pigmentation and melanocyte stem cells (MSC) which reside within the niche of hair follicle nodule and maintain hair pigmentation during subsequent growth cycles.
Melanoblasts are immature cells situated upon the epidermal basement membrane and with signals arising from adjacent cells, can differentiate into mature melanocytes.
Melanoblasts are essentially un-pigmented cells which comprise solely of immature melanosomes and are devoid of crucial enzyme of melanin synthesis – tyrosinase.
Dermal origin of melanoblasts is incompletely deciphered and understood.
Additionally, contribution of genetic environment which controls or preserves the evolution of cutaneous melanoblasts into melanocytes remains obscure [1,2].
Melanocytic activation is cogent to the site of solar lentigines and intercommunication with enveloping stromal environment. Specifically, iridial melanocytes tend to retain melanosomes within the cell cytoplasm as opposed to cutaneous and follicular melanocytes which predominantly transpose melanosomes to adjoining keratinocytes.
Melanocyte proliferation is persistent and perpetual in children and inhabit the enhancing cutaneous surface with progression into adulthood. In contrast, adult melanocytes gradually proliferate into completely differentiated cells and congregate within the basal epidermal layer. Thus, a communication is forged with circumscribing keratinocytes for transposition of melanosomes.
Contingent to epidermal localization, melanocytes and keratinocytes configure an “epidermal –melanin unit” where a single epidermal melanocyte adheres to approximately thirty or forty keratinocytes through dendrites. The communication permits transference of melanosomes from dendritic apices into encompassing keratinocytes with consequent determination of skin tone and safeguard against ultraviolet radiation induced skin damage [2,3].
Aforesaid concurrence amidst keratinocytes and melanocytes also permits an extensive correlation where keratinocytes can modulate cellular response of melanocytes. In fact, keratinocytes critically contribute in melanocytic reaction to ultraviolet radiation via specific ligands which impact mobilization of MC1R gene. Besides, keratinocytes are responsive and react to ultraviolet radiation, in contrast to melanocytes. Sequential to ultraviolet radiation exposure, keratinocytes delineate multiple ligands and generate specific reactive oxygen species (ROS) which are incorporated within melanocytes and engendered melanin pigment [2,3].
Disease pathogenesis
Spots of solar lentigines appearing on the face generally enhance with increasing age. Solar lenities are frequently associated with darker skin tone. Multiple senile solar lenities on the face are elucidated with Fitzpatrick skin type III and IV and are cogitated as the “lentigo aging pattern”. Aforesaid manifestations are due to an active melanocytic conformation exemplified in the skin types.
Configuration of solar lentigines is concurrent to sun exposure with resultant cutaneous photo-damage. Solar lentigines are concomitant to frequent sunburn and recreational exposure to sun, although occupational or lifelong sun exposure may not articulate solar lentigines. However, assessment of sunburns, degree of recreational sun exposure, acute photo-trauma or total lifetime sun exposure can be a challenging task.
Aetiology of solar lentigines is contingent to and diverse in various cutaneous regions.
Solar lentigines can be induced with specific therapies such as psoralens and ultraviolet A light (PUVA) and are cogitated as PUVA lentigines. Lesions can arise in psoriasis in concurrence with PUVA. Thus, solar lentigines can emerge in sun protected cutaneous surfaces. Atypical melanocytes with enlarged, hyper-chromatic nuclei are enunciated in an estimated 57% PUVA associated solar lentigines and around 70% in adjoining PUVA exposed skin devoid of lesions.
Bi-nucleated melanocytes and giant melanosomes can appear in individuals treated with PUVA with an evolving melanocytic dysplasia and malignancy. PUVA associated solar lentigines display melanocytes with elongated, numerous dendrites and an active melanogenesis.
Basal keratinocytes of PUVA induced solar lentigines demonstrate frequent, enlarged, singular melanosomes [3,4].
Disease characteristics
• Appear beyond 50 years of age, with cumulative photo-trauma and are stable lesions devoid of seasonal variation.
• Sun exposed skin such as face, hands, forearms, chest, back and shins is implicated.
• Pigmented spots vary betwixt millimeters to centimetres in dimension.
• Lesions are innumerable (few to hundreds), light yellow to dark brown with a well-defined perimeter.
• Caucasians and Asians with Fitzpatrick skin type I – III are commonly implicated.
• An environmental aetiology is cogent.
• Melanocytes are numerically enhanced up to 2.2 times.
• Magnitude of melanocytes and melanosomes is normal as is the quantification of melanosomes.
• Epidermal pigmentation is enhanced and rete ridges are elongated.
• Adjunctive features include melanosome complexes appearing in keratinocytes, enhanced quantification of mitochondria, mature endoplasmic reticulum, “pendulum melanocytes” and micro-invaginations into keratinocytes [1,2].
Clinical elucidation
Solar lentigo or solar lentigines primarily arise in elderly patients. Frequently, multiple, dark brown to black macules of magnitude 3 millimetres to 12 millimetres or more are cogitated. In contrast to ephelides, lenities are enlarged lesions with a dark brown discolouration and a magnitude of few millimetres to centimetres.
Pigment modification is frequent on the face, dorsum of hands and anterolateral forearm. It can emerge at various sites such as ventral torso, upper limbs, feet and shoulders and usually appears in skin with chronic sun exposure. Solar lentigines predominantly appear beyond 50 years of age and are unresponsive to seasonal variation. Solar lentigines can be quite enlarged and clinically simulate malignant melanoma [5,6].
Solar lenities can depict a metamorphosis to reticulated variant of seborrheic keratosis.
A differentiating feature of solar lentigines from common freckles is the persistence of lesions in the absence of sun exposure [4,6].
Molecular and genetic modifications
Solar lentigines are engendered from accumulated cutaneous photo-damage which induces genetic or epigenetic modifications in the genomic elucidation of keratinocytes.
Keratinocytes of solar lentigines regulate melanocytic growth, dendritic and melanogenic behaviour with enunciation of hyper-pigmented spots in concordance with altered micro environment and paracrine or cell to cell signalling communication. Articulation of hyper-pigmented spots is contingent to melanocytic genes incriminated in pigmentation.
RHC (red hair colour, fair skin, poor tanning ability) variant alleles of melanocortin-1-receptor (MC1R) gene enhances probable emergence of solar lentigines by 1.5 to 2 times. Functional elimination of MC1R variant alleles are associated with appearance of solar lentigines, thus incriminating melanocytes with declining MC1R activity in the configuration of solar lentigines [1,2].
Genomic variations of SLC45A2 are associated with solar lentigines.
SLC45A2 (Membrane Associated Transporter Protein) protein is a 12- trans-membrane - spanning protein of obscure function although it is possibly incriminated in melanin synthesis and it’s efficacy in melanocytes remains to be defined. Adjunctive proteins are additionally implicated in the formation of solar lentigines.
Genetic mutations of fibroblastic growth factor receptor 3 (FGFR3 17%) and phosphatidylinositol-4,5-biphosphate-3-kinase catalytic subunit alpha (PIK3CA 7%) genes are cogitated in instances of solar lentigines.
Endothelin 1 and endothelin B receptors are cogent for melanocyte development and solar lentigines demonstrate enhanced elucidation of genes in contrast to adjacent normal skin. Estimations are achieved by employing reverse transcriptase –polymerase chain reaction (RT PCR) and immune histochemistry [5,6].
KIT ligand (KITLG) or stem cell factor (SCF) is enhanced in solar lentigines. However, enunciation of receptor KIT can be within normal range.
Elevated expression of hepatocyte, keratinocyte and fibroblast growth factors are cogitated which influence genetic expression of melanocytes clustered in photo-traumatic cutaneous zones.
Skin implicated in solar lentigines depicts around 17 downgraded and 23 up-regulated genes and are devoid of pigmentation genes although categories of Wnt family genes, metalloproteases and inflammatory response genes are incriminated.
Solar lentigines can occur as a consequence of “pleiotropic syndromes” with secondary genetic factors.
Carney’s complex (CNC) arises due to mutation of protein kinase c- AMP dependent type-1 regulatory subunit alpha (PRKAR1A) gene which encodes the regulatory subunit 1 α of protein kinase A, an enzyme which is implicated in endocrine signalling pathway along with being a tumour suppressor gene.
Appearance of pigmentation is on account of protein kinase A activated signal transduction pathway accompanied by a down-regulation of melanocortin receptors.
Majority (85%) of subjects of LEOPARD syndrome demonstrate a missense mutation in the protein tyrosine phosphatase non-receptor type 11 (PTPN11) gene. PTPN11 gene encodes a member of protein tyrosine phosphatase and comprises of two tandem SRC homology-2 (SH2) domain and one protein tyrosine phosphatase domain.
Specific protein is a cytoplasmic signal transducer of multiple receptors for growth factors and hormones and functions by activating RAS mitogen activated kinase (MAPK) pathway [6,7].
Peutz Jeghers syndrome is engendered by mutations of serine/threonine kinase11 (STK11) gene. STK11 is denominated as a tumour suppressor gene and encodes for serine/threonine kinase which regulates cellular proliferation and polarity.
Nevertheless, it is indeterminate if aforesaid genetic constituents are implicated in the pathogenesis of solar lentigines [1,2].
Histological elucidation
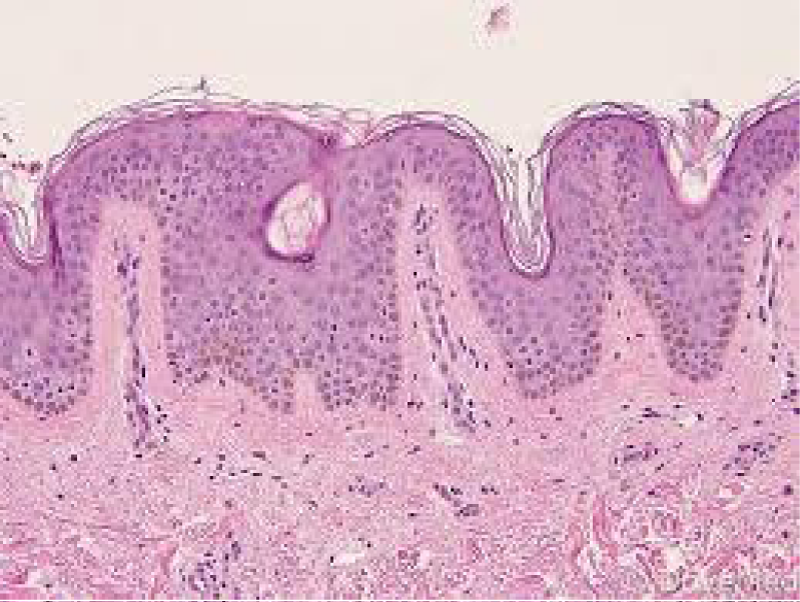
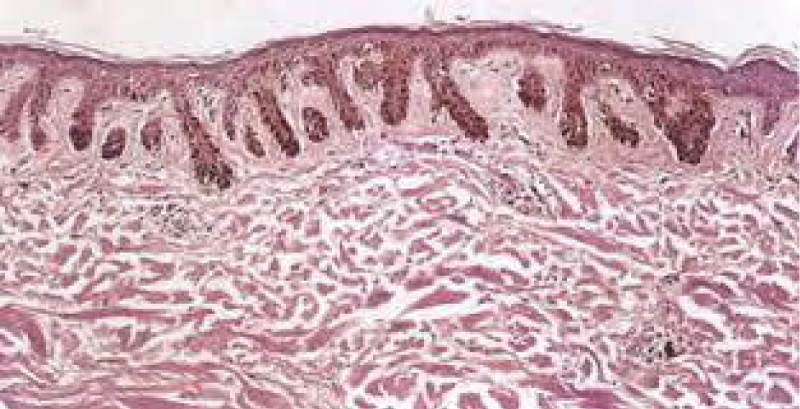
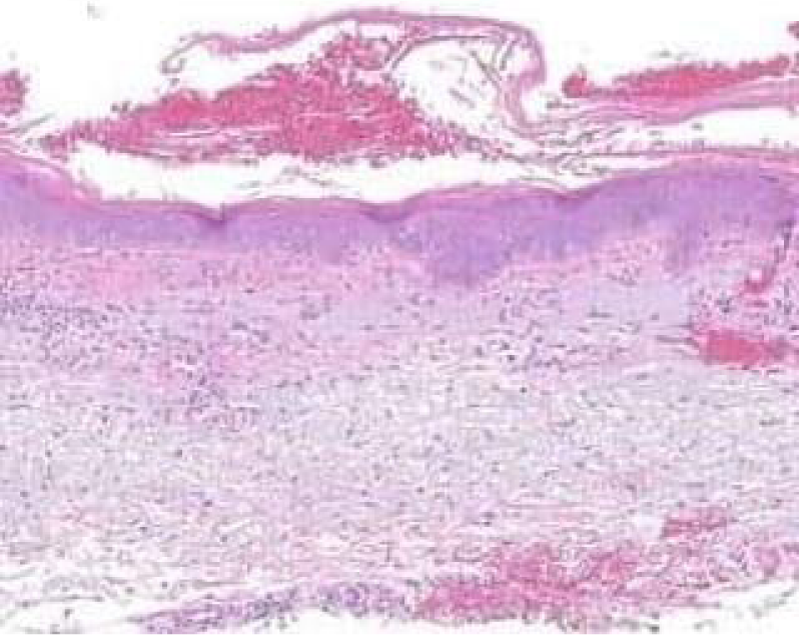
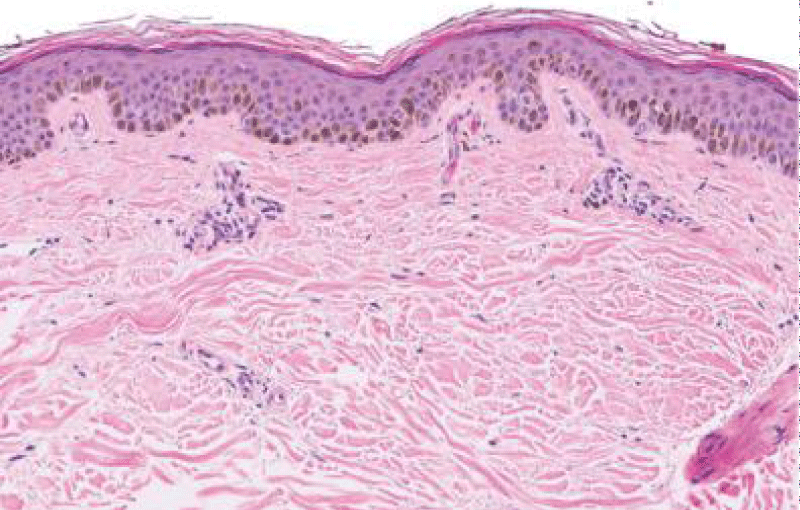
Typical morphology of solar lentigines enunciates an elongation of rete ridges with focally intense, basal hyperpigmentation. Solar elastosis is a constant feature.
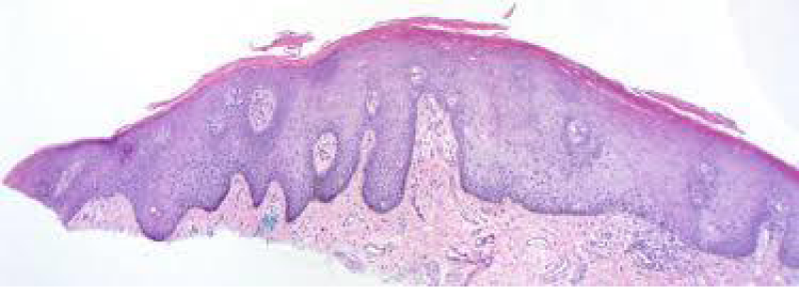
Spots of solar lentigines with hyper-pigmentation of basal layer and accompanying elongation of epidermal rete ridges also depict a compact, superimposed epidermis with keratinocytes which tend to accumulate melanin pigment.
Prominent proliferation of melanocytes and keratinocytes is cogitated within solar lentigines with consequent extension of basal layer and elongated epidermal rete ridges. Amplified length of rete ridges is not concordant with a perpetually elevated proliferation of solar lentigines. In fact, particular period of cellular proliferation of epidermal rete ridges in solar lentgines remains obscure [6,7].
Solar lentigines demonstrate three morphological subclasses.
• Class I depicts a flattened epidermal layer.
• Class II elucidates epidermal hyperplasia.
• Class III exhibits a combination of epidermal levelling and hyperplasia. Aforesaid manifestations are specific to distinctive zones such as face along with age of the subject and are indicative of progression of solar lentigines (Figure 1).
Figure 1: Solar lentigo with basal pigmentation and elastosis. Courtesy: Dovemed.com
A segregation on histology is necessitated from lesions such as lentigo maligna which demonstrates irregular nests and aggregates of melanocytes and a dissemination of singular melanocytes in the upper layers of epidermis [2,3].
Immune histochemical evaluation
Immune staining with Ki67 displays an absence of proliferative features amidst cutaneous lentigines and adjacent normal skin. Solar lentigines depict an elevated quantification of melanocytes within the epidermis, melanoblasts within infundibulum of hair follicles and melanocyte stem cells (MSCs) in hair follicle nodule. Elucidation of TYR protein within the melanocytes is enhanced. Melanocyte precursor cells are quantifiably amplified in solar lentigines with melanocytic activation [7,8].
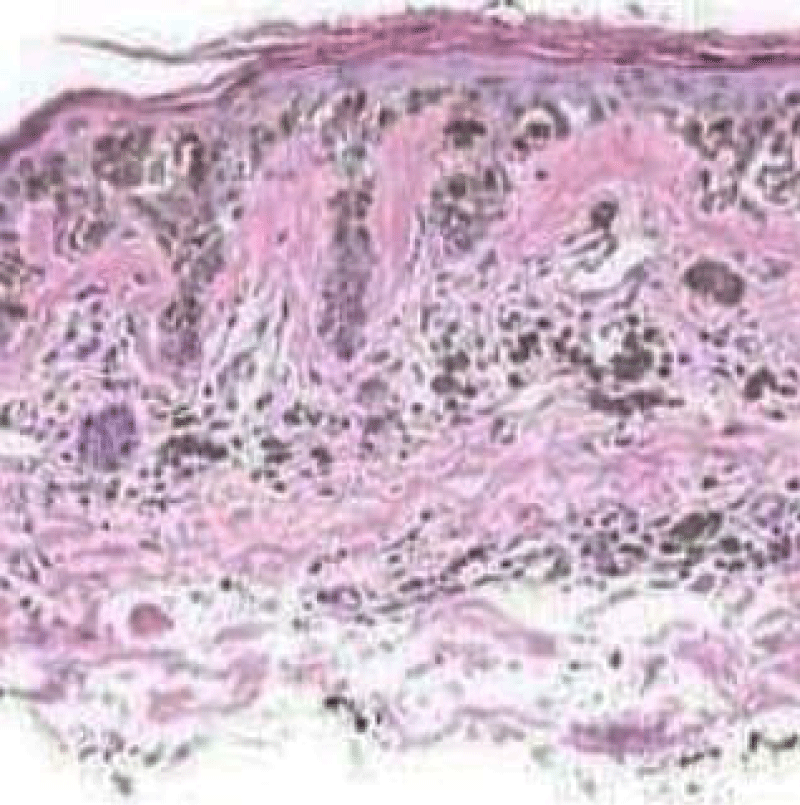
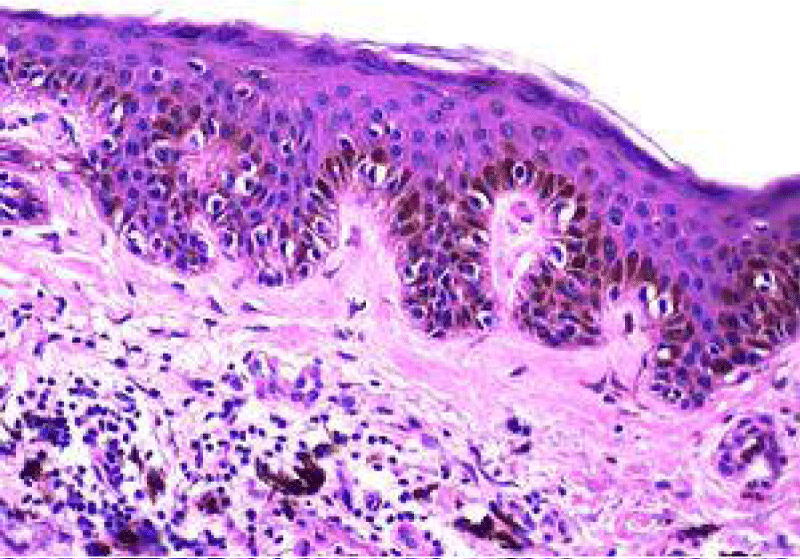
It can be surmised that melanocytes and precursor cells are responsive to signals arising from photo-damaged cutaneous surface whereas keratinocytes appear to initiate and develop the phenomenon. Keratinocytes demonstrate pigment accumulation, particularly in the perilesional skin, thereby implying that pigment concentration is an antecedent manifestation (Figure 2).
Figure 2: Solar lentigo with elongated rete ridges, abundant pigmented keratinocytes and parakeartosis. Courtesy: Dermatology advisor.
Immune reactivity for molecules such as S-100 protein, HMB-45, Melan A and tyrosine kinase is absent in pigmented keratinocytes, although melanocytes are appropriately discerned [7,8].
Electron microscopy
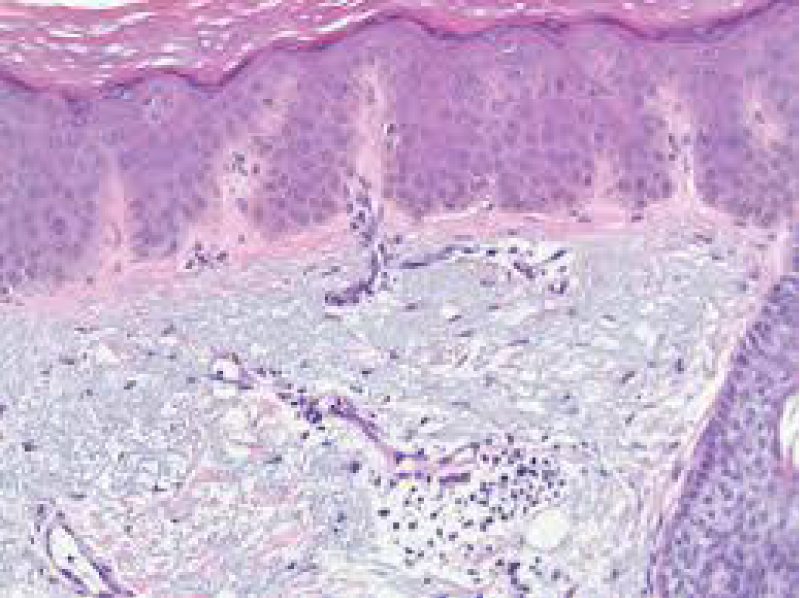
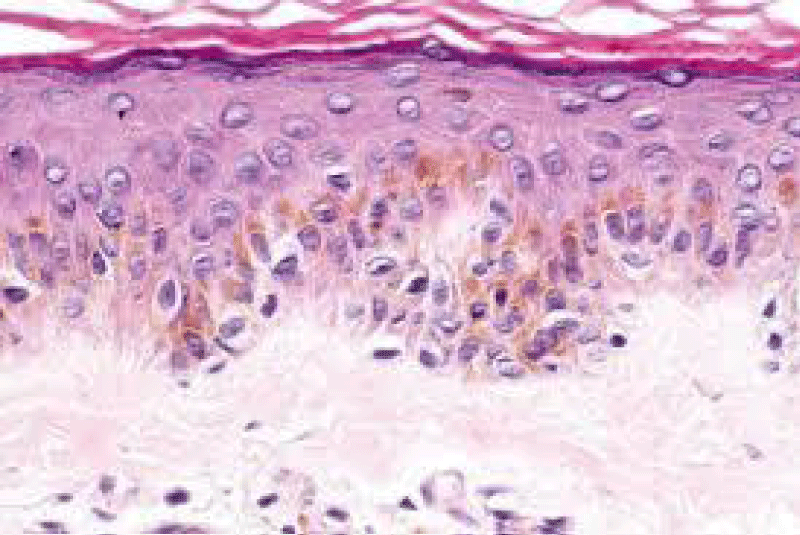
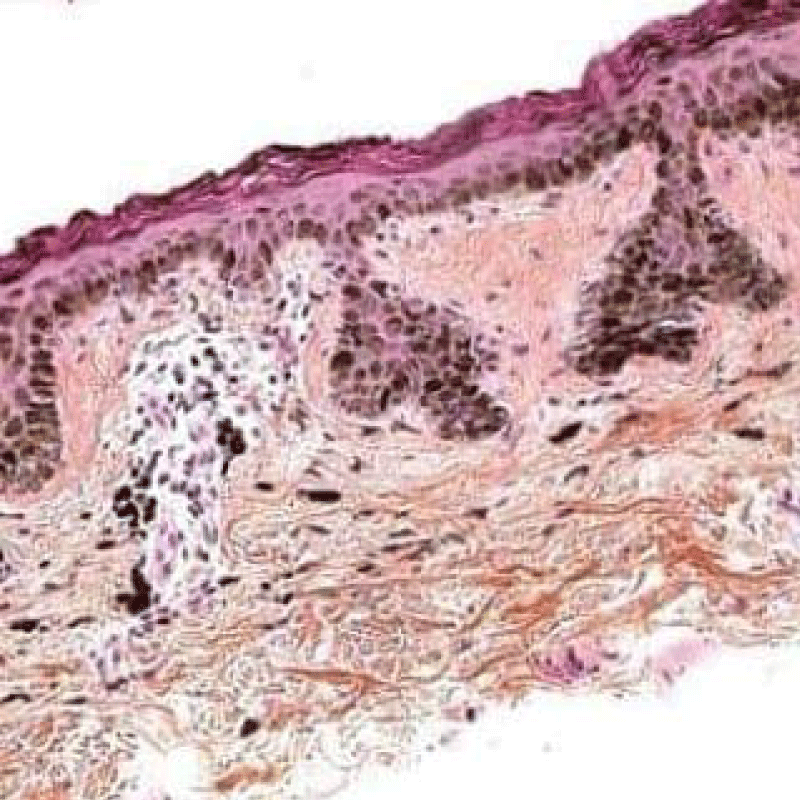
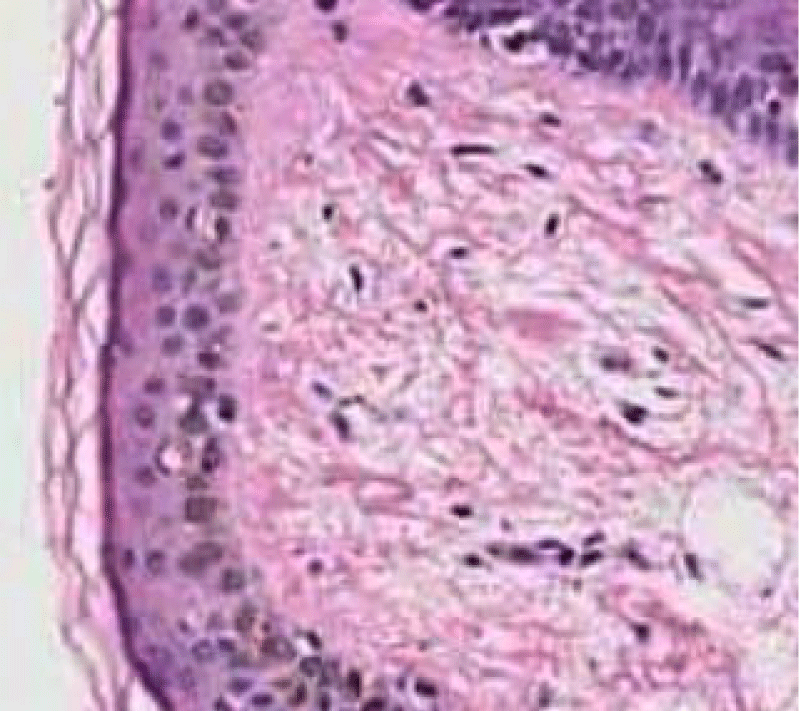
On ultra-structural examination, melanosomes appear to be of normal magnitude within the lentigines along with melanocytes of adjoining, non-involved cutis. Melanocytes of solar lentigines depict abundant mitochondria and a well delineated endoplasmic reticulum, contrary to the melanocytes of adjacent cutaneous region (Figures 3,4).
Figure 3: Solar lentigo with dispersed, pigmented keratinocytes , melanocytes and elongated rete ridges. Courtesy: Dermpedia.com
Figure 4: Solar lentigo with elongated rete ridges and intense, basal pigmentation. Courtesy: Dermamin.com
Basal keratinocytes of solar lentigines comprise of melanosome complexes with poly-melanosomes which configure mammoth pigment envelops positioned above cell nuclei, thereby indicating effective production and transposition of melanosomes from melanocytes to adjacent keratinocytes (Figures 5,6).
Figure 5: Solar lentigo with solar elastosis and basal, pigmented keratinocytes. Courtesy: Springer link
Figure 6: Solar lentigo with pigmented keratinocytes, melanocytes and epidermal flattening. Courtesy: Basic Medical Key
In solar lentigines, dermal- epidermal junction is discontinuous and disarranged and lamina densa is attenuated. Micro-invaginations arise into the keratinocytes and melanocytes extend into the dermis with articulation of “pendulum melanocytes”[9,10].
Therapeutic Options: Solar lentigines are therapeutically amenable to diverse categories of laser therapy which emit specific wavelengths absorbable by melanin. The chromophore melanin converts laser wave energy onto heat which exterminates the cutaneous pigment. Pulsed, pigment specific lasers are employed to selectively demolish the pigment in solar lentigo. Aforesaid treatment modality significantly enhances the assortment of therapeutic options (Figure 7).
Figure 7: Solar lentigo with epidermal hyperplasia and basal pigmentation. Courtesy: Basic Medical Key
Laser therapy eliminates excessive pigment and is devoid of alteration of surrounding skin. Sequential to laser therapy, a fresh layer of non-pigmented keratinocytes are generated with induction of healing (Figures 8,9).
Figure 8: Solar lentigo with elongation of rete ridges and intensely pigmented keratinocytes. Courtesy: Mungfall.com
Figure 9: Solar lentigo with epidermal hyperplasia, elastosis and basal pigmentation. Courtesy: Science direct
Specified treatment is associated with minimal complications and enhanced patient compliance. Lasers are considered as an efficacious methodology for treating solar lentigines. Short nanosecond pulse duration Q switched Nd YAG lasers are considered superior to fractional carbon dioxide laser for managing solar lentigines, although adoption of aforementioned laser is painful and requires an extended application for post-therapeutic alleviation [10,11].
Laser is contemplated as preferential therapy, in contrast to frequently employed modalities such as cryotherapy or skin peelings with lightning. Eradication of solar lentigines with nanosecond and pico second switched lasers is a quick and practicable treatment modality with minimal sessions (Figure 10).
Figure 10: Solar lentigo with basally located, pigmented keratinocytes, melanocytes and projection of rete ridges. Courtesy: Librepath.com
Biophotonic treatment incorporates a multi light emitting diode (LED) lamp accompanied by a specific gel constituted of chromophores. Aforesaid combination emits a fluorescent light energy which augments the cutaneous countenance. Eradication of wrinkles and fine lines, diminished magnitude of skin pores, reduction in inflammation and improved alleviation by activating collagen formation are achieved with superior long term therapeutic outcomes [9,11].
Concurrence of laser and biophotonic technique in order to specifically ablate the pigmented spot is beneficial and can be adopted. Application of laser in combination with biophotonic tissue stimulation is competent in eradicating solar lentigines along with general rejuvenation and enhancement of skin appearance (Figure 11).
Figure 11: Solar lentigo with epidermal flattening, solar elastosis and mild basal pigmentation. Courtesy: You tube
Thus, biophotonic treatment can potentially be conjoined with laser and adjunctive invasive therapies. Cogent anti-inflammatory actions which regulate the skin and augment collagen with resultant skin rejuvenation can be obtained. The treatment is devoid of photosensitivity and can be employed in each season [10,11].
- Praetorius C, Sturm RA, Steingrimsson E. Sun induced freckling: ephelides and solar lentigines. Pigment Cell Melanoma Res. 2014: 27; 339-50. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24517859
- Scarcella G, Dethlefsen MW, Nielsen MCE. Treatment of solar lentigines using a combination of picosecond laser and biphotonic treatment. Clin Case Rep. 2018: 6; 1868-1970. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30214780
- LaRosa CL, Foulke GT, Feigenbaum DF, Cordoro KM, Zaenglein AL. Lentigines in resolving psoriatic plaques: rarely reported sequelae in paediatric cases. Paediatr Dermatol 2015: 32; e114-e117. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25727728
- Schoenewolf NL, Hafner J, Dummer R, Bogdan Allemann I. Laser treatment of solar lentigines on dorsum of hands: QS ruby laser versus ablative CO2 laser fractional laser- a randomized controlled trial. Eur J Dermatol. 2015: 25; 122-126. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25788397
- Vachiramon V, Panmanee W, Techapichetvanich T, Chanprapaph K. Comparison of Q switched Nd: YAG laser and fractional carbon dioxide laser for treatment of solar lentigines in Asians. Laser Surg Med. 2016: 48; 354-359. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27096729
- Ezzedine K, Mauger E, Latreille J, Jdid R, Malvy D, Gruber F, et al. Freckles and solar lentigines have different risk factors in Caucasian women. J Eur Acad Dermatol Venereol. 2013: 27; e345-e356. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22924836
- Hafner C, Stoehr R, van Oers JM, Zwarthoff EC, Hofstaedter F, et al. FGFR3 and PIK3CA mutations are involved in the molecular pathogenesis of solar lentigo. Br J Dermatol. 2009: 160; 546-551. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19076977
- Horvath A, Stratakis CA. Carney complex and lentiginosis. Pigment Cell Melanoma Res. 2009; 22: 580-587. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3136757/
- Kovacs D, Cardinali G, Aspite N, Cota C, Luzi F, et al. Role of fibroblast derived growth factors in regulating hyperpigmentation of solar lentigo. Br J Dermatol. 2010: 163; 1020-1027. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20662835
- Praetorius C, Grill C, Stacey SN, Metcalf AM, Gorkin DU, et al. A polymorphism in IRF4 affects human pigmentation through tyrosine dependent MITF/TFAP2A pathway. Cell. 2013; 155: 1022-1033. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24267888
- Yamada T, Hasegawa S, Inoue Y, Date Y, Arima M, et al. Comprehensive analysis of melanogenesis and proliferation potential of melanocyte lineage in solar lentigines. J Dermatol Sci. 2014; 73: 251-257. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24314758